3D printing with a bite
In the early days of additive manufacturing (AM), dentistry seemed a natural application. The three classes of materials most commonly used in dental appliances – ceramics, metals and plastics – coincide with those most commonly used in AM. This technique is also well-suited to creating bespoke products with complex geometry to high levels of accuracy. However, despite the initial enthusiasm, there was very little uptake.
The reasons lie in the network of dental laboratories that support dentists and their patients, by producing the crowns, copings, bridges, plates and dentures that dentists prescribe. Often small and employing skilled metal workers and ceramists, these laboratories have developed an understanding of the materials and techniques that meet the exacting standards required for dental products, embracing strength, accuracy, feel, appearance and affordability. Dentistry has a reputation for conservatism, an understandable reluctance to adopt new materials or techniques unless and until the benefits are proven.
engineers worked with the laboratories, not to supplant their existing methods, but to understand how their traditional techniques worked, and where and how AM could best be introduced to improve them
So, engineers worked with the laboratories, not to supplant their existing methods, but to understand how their traditional techniques worked, and where and how AM could best be introduced to improve them.
Computer-designed dentures
As recently as the mid-2000s, the principal materials in a dental lab were gypsum plaster, carving wax and casting alloys. A flexible, negative mould (impression) of the patient’s upper or lower teeth would arrive from the dentist, and would be used to make a ‘positive’ plaster-cast model. A craftsman would then use this to create the shape of the required crown or coping in wax, which would then be cast in metal using the ‘lost wax’ method, a casting technique that may be more than 5,000 years old. Around the mid-2000s, dental labs began introducing CAD (computer-aided design) systems to scan their dental models and design crowns or copings in their own facilities, with a centralised milling factory manufacturing the items.
Around the mid-2000s, dental labs began introducing CAD (computer-aided design) systems to scan their dental models and design crowns or copings in their own facilities, with a centralised milling factory manufacturing the items
Metal alloys continued to be used, but often the materials of choice became soft-state aluminium oxide (alumina) or zirconium dioxide (zirconia), subsequently sintered to produce a very hard chewing surface. Both ceramics can deliver an aesthetic, tooth-like appearance.
Over the following years, dental milling machines, ceramics and tooling have all improved and dropped in price, making metal-free aesthetic restorations affordable. However, metal alloys are still the predominant materials, with a compelling blend of affordability, suitable mechanical characteristics and evidence in use.
What is additive manufacturing?
🖨️ Borrowing technology from the printing industry to create 3D objects and the pros and cons of AM
Additive manufacturing is the creation of three-dimensional objects from a three-dimensional digital model by successively placing thin layers of material on top of one another. It can create objects of almost any shape using a wide variety of materials, particularly polymers, ceramics and metals.
A designer can create digital models directly on a CAD system, or a three-dimensional image of the prototype is captured using a variety of forms of digital photography or light scanning, or (for example, for a human organ) a CT scan (multiple X-rays) or an MRI scan.
Some early applications borrowed technology from the printing industry, including inkjet printer heads, to build up solids using suitable binder materials – hence the term ‘3D printing’. A low-cost market developed, using mostly thermoplastics extruded through nozzles, to create objects varying from toys and jewellery to electronics packaging and simple architectural models. Most were small-scale, but larger applications included manufacturing formwork panels in the building industry to create exposed concrete finishes with elaborate geometry.
Meanwhile at the industrial end, development focused on the use of an energy source such as a laser or electron beam to fuse polymeric or metallic powders layer by layer. Initially, this created ‘rapid prototypes’ of parts quicker and cheaper than conventional methods. The term ‘additive manufacturing’ (AM) emphasises the contrast to traditional ‘subtractive manufacturing’, for example carving a natural material, or milling and drilling a casting.
Potential advantages include the ability to manufacture on-demand, rather than carry large stocks; to displace expensive machinery and tools such as milling machines; and to make products that would otherwise be too expensive to manufacture.
Some products would be impossible to create by casting or machining. These include products with complex internal channels and pathways, which would otherwise have to be created by assembling multiple parts or intricate lattices and honeycombs that can replicate natural materials, such as bone.
AM can reduce the weight of structures by as much as 40%, which is particularly important in the aerospace and automobile industries where weight reduction is crucial.
However there have also been drawbacks that have slowed AM’s introduction, including the high investment cost, early limitations on the conventional CAD software that imposed design and quality drawbacks, constraints on suitable materials, and advances in competing technologies.

Recent advances in AM include the development of load-bearing lattice structures for orthopaedic implants. By incorporating a lattice, implants can provide a scaffold structure for new bone tissue to grow into, with the spacing and strut thickness of the lattice optimised to match the stiffness of surrounding bone. The lattice, with its ‘pseudo-random’ internal structure, would be near impossible to manufacture in any other way than AM © Renishaw
Material replacements
How could AM enter this well-established and fast-developing industry? The first issue was problems with CAD systems. Early systems usually used a proprietary format only readable with software developed by the original CAD vendor. This made it difficult to transfer data to the manufacturing systems, which were also not good at replicating non-uniform shapes and surfaces. The second issue was that the CAD files can have imperfections, and AM is particularly intolerant of errors in files. The issue of proprietary data files was solved between 2009 and 2010 as CAD programs became more open and could output a universally readable file format (in this case, STL). They also became more capable of replicating complex three-dimensional geometry. The challenge of providing data suitable for AM was addressed by improved software in all stages of the process, and including an ‘auto-healing’ feature to detect and correct errors.
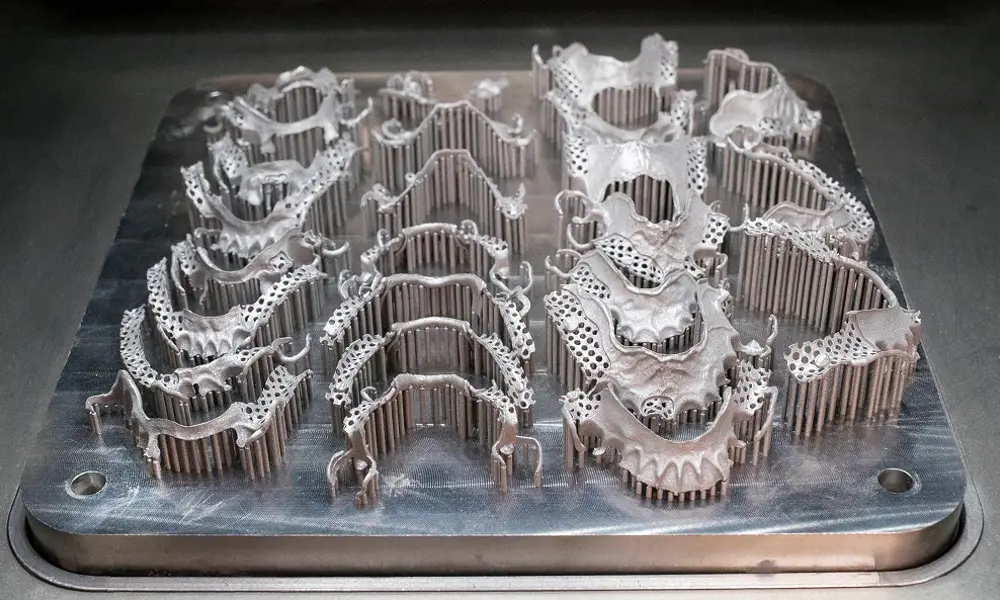
Additive manufacturing can capture the complexity of removable partial dentures © Renishaw
Could AM replace the milled ceramics for new teeth? Zirconia, for example, is expensive and cannot easily reproduce the translucence of natural teeth (or of dental porcelain). It can also obscure subsequent decay beneath the crown, and – despite its great strength – can be subject to micro-cracking. However, AM technology is not yet sufficiently advanced for practical application for ceramic teeth. Skilled ceramicists still create the finest aesthetic finishes, painting multiple layers of dental porcelain up to 1.5 to 2 millimetres thick over a metal (or zirconia) base and firing it in an oven to produce an excellent bond. This results in a strong and resilient tooth with a porcelain finish that can exactly match the colour and translucency of the patient’s existing teeth.
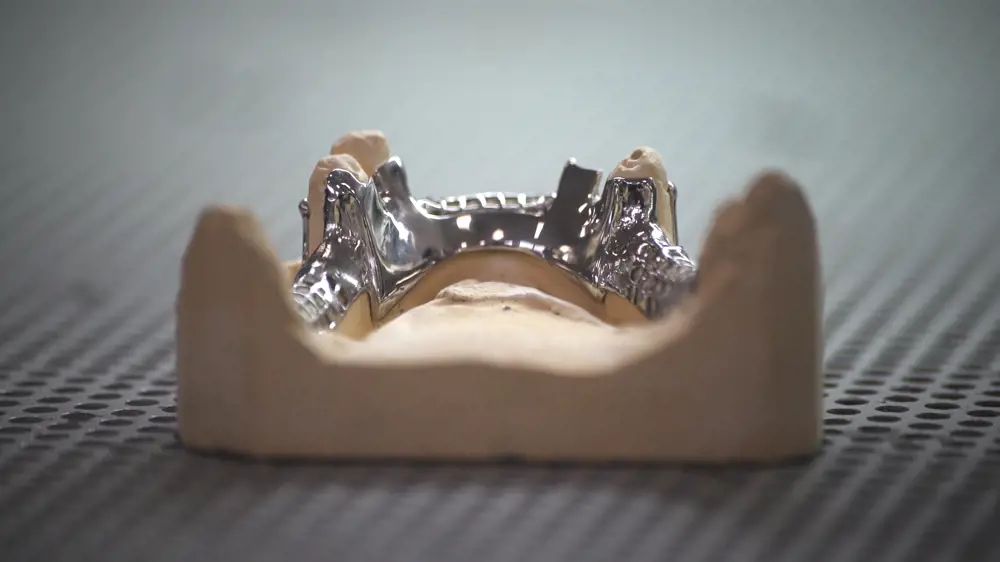
The focus moved to the metal parts. A crown, for example, may be made in metal, with the underside fitting snugly on the remains of the tooth that the dentist has shaped to form a foundation, and the upper part ready for the ceramist to add the porcelain coating
The focus moved to the metal parts. A crown, for example, may be made in metal, with the underside fitting snugly on the remains of the tooth that the dentist has shaped to form a foundation, and the upper part ready for the ceramist to add the porcelain coating. Larger items include bridges and denture frames. Dental labs were already equipped with CAD, which made the adoption of AM easier. An added driver was the shortage of skilled metal-working technicians and the escalating wages of those remaining: converting some of the metalwork to AM made business sense.
To be suitable for dental work, the material must be resistant to corrosion, acceptable by the human body, comfortable in the mouth and affordable. Its mechanical characteristics include a combination of strength, elongation, elasticity and resistance to fatigue. Casting alloys have gone through a long process of natural selection to come up with the right mix of properties. Cobalt chrome and nickel chrome became the most widely used, and labs had invested heavily in compatible porcelains so an unnecessary change was undesirable. The latter became the alloy of choice for low-cost dental frameworks as it is easy to cast; although nickel sensitivity in a minority of patients has become an issue. Titanium has excellent biocompatibility and is used extensively in the manufacture of dental implants, but dental labs find it very difficult to cast due to its low density and it can be hard to get porcelain to adhere to it.
To be suitable for dental work, the material must be resistant to corrosion, acceptable by the human body, comfortable in the mouth and affordable
The metal that best matches the labs’ requirements is cobalt chrome (CoCr), using laser powder-bed fusion (LPBF) AM technology. Within an LPBF system, a thin layer of CoCr powder is deposited as thin as 20 microns thick, on the machine’s build plate and a super-fine laser beam melts an area of the powder corresponding to a 2D slice of the CAD model. The build plate moves down, another layer of powder is added and the process repeats until the component is complete. The unfused powder is then removed and captured for re-use. For efficiency, labs will usually manufacture many items simultaneously, each with its own disposable identity tag.
The metal that best matches the labs’ requirements is cobalt chrome (CoCr), using laser powder-bed fusion (LPBF) AM technology
AM-produced metal parts were originally considered too brittle for larger items such as removable dental frames, which are still used extensively where implants or bridges are either clinically unsuitable or unaffordable. However, the manufacture in an inert, argon atmosphere with less than 0.1% oxygen and a careful post-production heat-treatment process have helped to overcome this process.
The other principle objection is the high initial cost of an AM machine – around £250,000 to £500,000 – which smaller labs can address by collaborating to create the volume to bring cost-per-part down and justify the investment. Quality and accuracy have been found to match established materials and techniques, and in some respects (such as fatigue resistance for dentures) produce substantial improvements. Furthermore, AM provides better quality assurance and,
by reducing faulty products, cuts scrap rates and the need for ‘re-working’. It also substantially reduces material wastage, can reduce turnaround time for dentists, and can use the time of skilled technicians more efficiently.
Bone substitutes
Additive manufacturing is also helping with maxillofacial surgery, a specialism of dentistry dealing with the bone and soft tissue around the jaw and face.
A couple of years ago an oral cancer patient at the University Hospital of Wales in Cardiff required surgery to remove the left side of his lower jaw. The surgeon and his team proposed to use bone from the patient’s fibula – a bone in the lower leg which carries little load in an adult and is therefore suitable for ‘harvesting’ for such purposes – to construct a new jaw which could be attached to the remaining healthy bone, and to which new teeth could be added. AM offered a way of maximising accuracy while minimising both risk and time spent in the operating theatre.
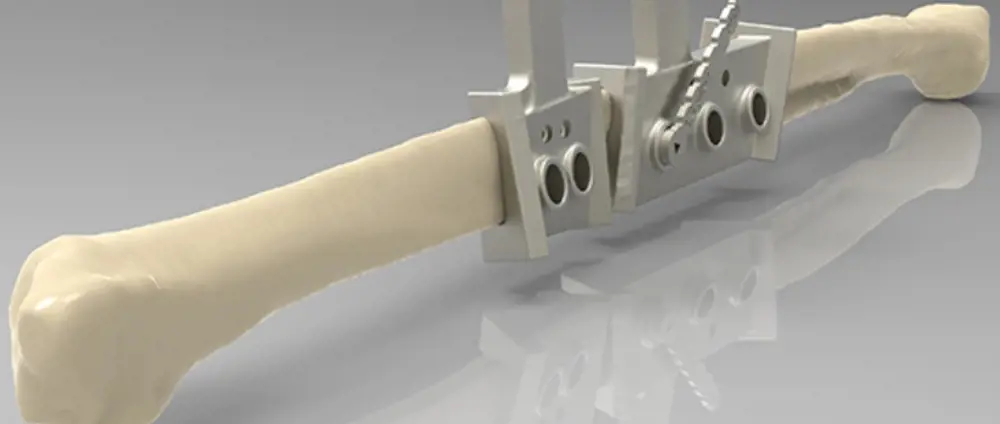
The AM-produced cutting-and-drilling guide enabling the surgeon to accurately cut and pre-drill sections of fibula for building the new jaw © Renishaw
CT scans produced accurate three-dimensional models of both jaw and fibula. This allowed the team to design the new jaw using two parts of the fibula assembled and attached to remaining bone, with mitred joints to create the shape. AM was used to produce a metal plate to hold the parts together and attach them to the two remaining healthy sections of the jaw.
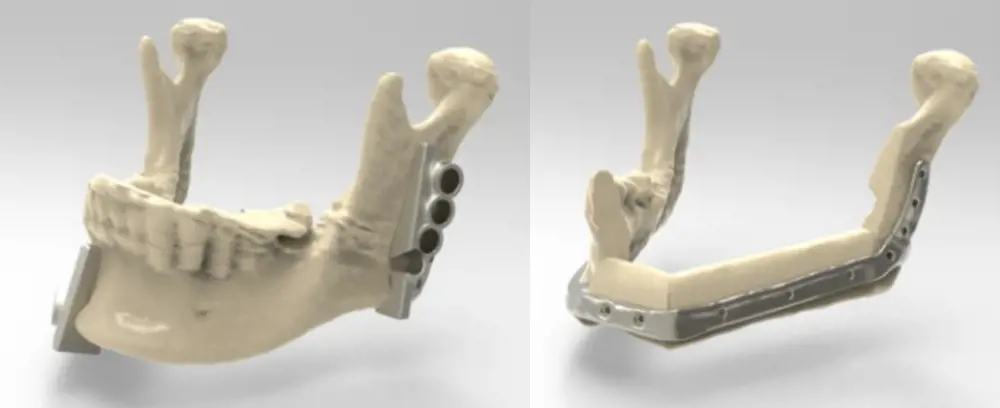
Two guides enabling the surgeon to cut out the infected middle section of the jaw, and pre-drill holes for the plate connecting to the new jaw (left). The new jaw, with the two sections of bone from the fibula connected to the remaining jaw either side by an AM-produced titanium plate (right) © Renishaw
The next part was a particularly ingenious innovation. After the surgeon had located the best target section of the fibula flap to harvest, a cutting guide was designed to help him remove, with precise control, two sections of bone and vascular connective tissue. The contoured guide, with its complex three-dimensional geometry matched to the CAD model of the bone, fitted the fibula in only one location, removing any possible error.
The guide also included holes that precisely located where to drill pilot holes matching the screw holes in the plate, so that the new jaw could be assembled quickly and accurately. Two further cutting-and-drilling guides were produced to precisely fit the ends of the remaining jaw, allowing these too to be accurately cut and drilled. Both the guides and the plate were manufactured using AM, and in both cases the material was titanium.
Removal of the section of fibula, and building and inserting the new jaw were completed in a single operation, with great success. Within weeks the patient was getting married and was delighted with the operation. Some 25 further operations using the same technique have followed.
Aerograft
🦴 The winning synthetic bone graft innovation
The Royal Academy of Engineering’s Enterprise Hub provides support for budding entrepreneurs in several ways. Its annual Launchpad Competition identifies and supports talented engineering entrepreneurs aged 16 to 25, while its Enterprise Fellowships scheme does the same for the founders and leaders of tomorrow’s high-tech companies.
In 2014, Dr Niall Kent, who has a doctorate from dental school, and his colleague Dr Alessia D’Onofrio won the first Launchpad Competition and JC Gammon Award. Their winning innovation is a synthetic bone graft material that can be used to improve the stability of implants in a range of dental and orthopaedics procedures. This provided £15,000 to catalyse growth of their startup company, mentoring from Academy Fellows, and regular meetings with the Award’s principal benefactor, investment manager David Gammon HonFREng. Two years later, Niall’s co-researcher at UCL, chemical engineer Dr Silo Meoto, received an Enterprise Fellowship to further develop a dental bone graft called Aerograft. These Fellowships provide up to £60,000 in funding plus mentoring, access to the Academy’s networks, and training in essential business skills.
The material is a bioactive calcium phosphosilicate, which, in solution, releases calcium and phosphate ions that precipitate to form hydroxylapatite, a mineral found naturally in bones and teeth. It integrates with the existing host bone, encourages natural growth and (in time) naturally disintegrates leaving strong, new bone.
Bone tends to wither unless subject to constant stress, and dental implants need to be embedded on sufficient and strong bone. Bone graft materials are used to regrow bone where insufficient bone is present for an implant fitting. Current bone graft substitutes, such as bovine bone, and synthetic materials, such as ceramic, have limitations. Aerograft offers a higher integration rate (how well the body accepts it) and can also be ‘tuned’ to produce the different resorption rates (the time taken to be naturally removed by the body).
Aerograft is half way through the extensive (and expensive) tests needed as part of the CE approval process required to sell products in the EU, with encouraging results so far. Negotiations are underway to secure additional funding and partnerships. Meanwhile, a company is being formed with Niall as CEO and Silo as chief technical officer.

Aerograft is a bioactive aerogel sillicate-based material with calcium and phosphate ions. The material is used to improve the stability of implants in several dental and orthopaedic procedures
Manufacturing teeth
AM is now firmly entrenched in dentistry, working alongside long-established craft-based techniques. This is an example of how beneficial, evolutionary change can be achieved by cross-fertilisation between an established industry and the providers of new technology.
Further adoption of AM is likely to follow. Already 3D-printed plastic models, created with help from a new breed of handheld intraoral scanners operated by dentists, are beginning to replace traditional plaster models. Within five years, AM technology may have advanced sufficiently, and the cost reduced, to allow AM-produced ceramic teeth to replace casting and milling. AM is also making progress in the related fields of maxillofacial surgery and orthopaedic surgery. Its application in printing patient-specific implants, protheses and medical guides could enable surgeons to deliver life-changing procedures that otherwise might not have been possible.

Creating the study model for the dental prothesis
Manufacturing a dental prothesis
To create the study model, the dentist takes an impression, most commonly in silicone. The impression is posted to a dental lab which creates a study model by pouring gypsum plaster into the impression. Segmentation of individual teeth helps subsequent activities. Finally, a lab scanner digitises the model, often using structured light scanning.
The impression is posted to a dental lab which creates a study model by pouring gypsum plaster into the impression

The design file to a finished crown
For the design and build preparation, digitised data is imported into a dental CAD package where the tooth restoration is designed using the relationships of the adjacent and opposing arrangements of teeth as an aid in the lab. The design data is sent to the dental manufacturer and prepared for build. This is where the build orientation is determined, ID tags and build supports are added to each part and, finally, the parts are nested.
For the production, the build file is then used to manufacture parts at the dental manufacturer. There could be 200 to 300 parts on one plate. The framework is shipped to the lab where it may be placed on the model.
***
Hugh Ferguson spoke to Ed Littlewood, Marketing Manager of the Medical Dental Product Division, and Alex Harris, Applications Engineer, both from Renishaw, and Dr Silo Meoto from Aerograft to write this article.
This article has been adapted from "3D printing with a bite", which originally appeared in the print edition of Ingenia 75 (June 2018).
Contributors
Hugh Ferguson
Author
Keep up-to-date with Ingenia for free
SubscribeRelated content
Design & manufacturing

Super cool(er)
Welsh startup Sure Chill has developed a cooler that uses the properties of water to keep its contents cool for around 10 days without electricity. This is ideal for storing items such as vaccines where electricity sources are unreliable.

R&D investment makes good business sense
In just five years, Dr Ralf Speth FREng has presided over a revolution in design and manufacturing that has helped create a new family of engines and has overhauled Jaguar Land Rover (JLR) production facilities.

Steel can arise from the ashes of coal
Thousands of people were laid off in the UK steel industry in 2015 and there are pessimistic future forecasts. Professor Sridhar Seetharaman of the Warwick Manufacturing Group argues that smaller, flexible steel mills implementing new technology would better cope with fluctuating global trends.

Integrating metrology in business and academe
Professor Jane Jiang’s interest in measuring began when she worked on a bus production line in China. She found that the best way to improve quality, consistency and productivity was through metrology, the science of measurement. Today, she runs the UK’s largest metrology research group.
Other content from Ingenia
Quick read

- Environment & sustainability
- Opinion
A young engineer’s perspective on the good, the bad and the ugly of COP27

- Environment & sustainability
- Issue 95
How do we pay for net zero technologies?
Quick read

- Transport
- Mechanical
- How I got here
Electrifying trains and STEMAZING outreach

- Civil & structural
- Environment & sustainability
- Issue 95