Engineering cells to grow tissue
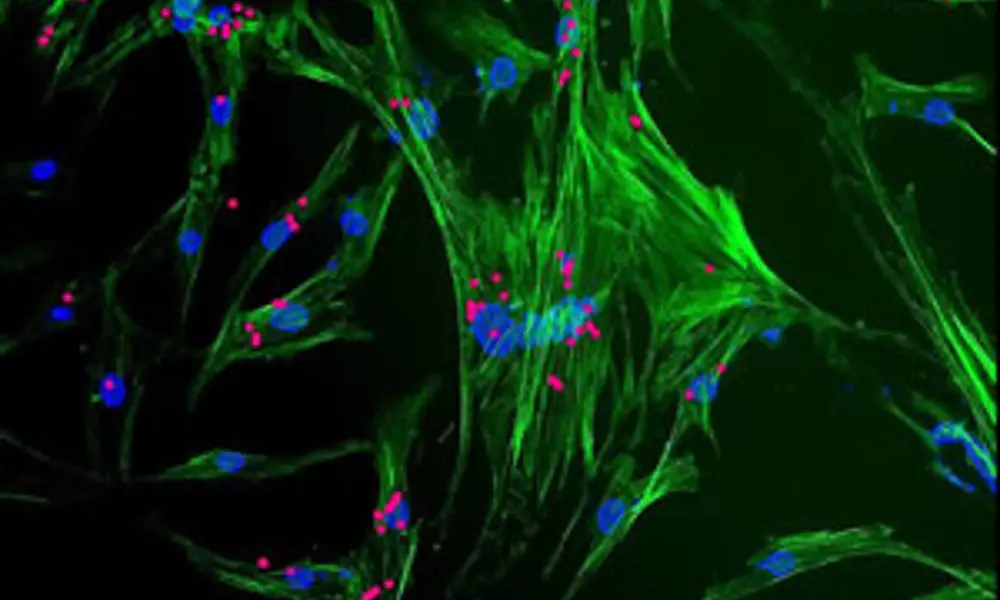
Stem cells (green and blue) with nanoparticles (magenta) viewed through a microscope. The nanoparticles are used to control cell behaviour © Professor El Haj
A new field of medicine that harnesses the cells in the human body to repair tissue damage and treat disease is gathering momentum. Cell therapy is already being used to treat osteoarthritis and osteoporosis, as well as heart disease, multiple sclerosis and dementia. Ultimately, tissue engineers hope to regenerate major tissue and grow organs, removing the need for reconstructive surgery and transplant of donor organs. Ingenia talked to Professor Alicia El Haj of Keele University to find out what different therapies can do right now and how her research is paving the way to innovative treatments.
In July 2011, surgeons in Sweden replaced a patient’s damaged trachea with a polymer-based version fabricated by researchers from University College London. Using 3D scans of the patient’s trachea and two main bronchi, the UCL researchers were able to model and build a replica of his windpipe.
They soaked the replica, a porous polymer scaffold, in a solution of stem cells taken from the patient’s bone marrow and then placed the ‘seeded’ scaffold in a growth chamber or bioreactor to grow bone tissue. Within just two days the cells were able to divide and grow, a process known as cell differentiation common in tissue repair, converting the inert polymer to a biological composite material ready for transplant. This operation marked the world’s first synthetic tracheal organ transplant.
Progress in the field of cell therapy has been rapid over the last 15 years. In 1998, James Thomson from the University of Wisconsin, USA, isolated human embryonic stem cells, showing their potential to rejuvenate and to specialise into tissues. Today, adult-derived stem cells are being used to treat myriad conditions from premature ageing and the menopause to osteoarthritis, multiple sclerosis, dementia and heart disease (see table: Conditions treated with cell therapy).
While the UK’s regulatory framework on using embryos and other human cells has helped put the nation’s researchers, including Professor Alicia El Haj, at the forefront of the field, progress was only made thanks to organised research efforts. In his 2007 report Race to the top, Lord Sainsbury highlighted how universities such as Keele, Nottingham and Loughborough were working together to create major centres to drive regenerative medicine forward. And early last year the ESPRC Centre for Innovative Manufacturing in Regenerative Medicine was established, comprising the same universities and aiming to pool research expertise and promote commercially robust practices and processes.
Tissue engineering
The recent synthetic trachea transplant is just one example of treatments that are taking place worldwide, thanks to pioneering research from Professor El Haj and fellow tissue engineers. El Haj is founding Director of the Institute of Science and Technology in Medicine at Keele University, an institute that has already treated hundreds of osteoarthritis sufferers. Osteoarthritis is the most common form of joint disease, and El Haj and colleagues have been using their cutting-edge cell therapy techniques to treat patients.
During this treatment, cartilage cells are taken from a healthy part of the patient’s knee and cultured in the laboratory, or in vitro, for several weeks in order to grow new knee tissue. The tissue is then implanted in the damaged joint, replacing the worn cartilage. More recently, clinical colleagues are commencing a trial to use patients’ cartilage cells mixed with bone marrow stem cells to see if combining the two in the same process is more effective.
Professor El Haj’s techniques are not just limited to bone and joint repair. Her cell therapies could be used to repair tissue damage in other parts of the body and are paving the way to a host of novel, ground-breaking treatments. Stem cells could be injected into the body to repair any tissue or organ. Cultured stem cells could grow a synthetic liver or kidney, and a stem cell bank could be set up where cells donated by anyone could be processed and delivered to a patient’s bedside. While these treatments are not with us yet, breakthroughs from El Haj and colleagues are bringing us closer.
In the beginning
El Haj’s first breakthrough took place some 10 years ago and involved developing a novel technique, based on magnetic nanoparticles, to control how cells communicate with neighbouring cells, a necessary step towards tissue growth. Working with Professor Jon Dobson, an expert on biocompatible magnetic nanoparticles, she started looking at how magnetic nanoparticles could be used to control cell activity and grow tissue.
Together they developed and patented a process to tag these magnetic nanoparticles to specific ion channel receptors on a stem cell, the protein molecules on a cell membrane that receive chemical signals from neighbouring cells. These signals might instruct the cell to divide or die, or allow certain molecules to enter or exit.
Crucially, the researchers discovered that they could use a small magnetic field to ‘pull’ the magnetic nanoparticle and open the ion channel receptor, and in doing so set off the chemical chain reaction that would lead to tissue growth (see Opening the door to tissue growth). Their breakthrough meant magnetic nanoparticles could now be used to activate receptors, control cell responses and initiate tissue growth at a time when nobody believed this was possible. Researchers patented this technique and have also spun out a company from Keele University, called MICA Biosystems. This aims to bring the technology to the clinic as well as commercialise in-vitro instrumentation tools for industry applications.
3D designs
The next step was to demonstrate that the technique could be used in a three-dimensional structure to grow tissue, and perhaps other artificial organs. At this time, several researchers had already been growing tissue on porous constructs but quite often these scaffolds would break during processing; could El Haj’s technique overcome this?
To grow tissue, researchers had been soaking their scaffolds in a stem cell solution and then placing their ‘seeded’ scaffolds in specially-designed growth chambers, known as bioreactors (see Growing organs in vitro). To stimulate the stem cells to grow tissue, mechanical forces would be applied to the seeded scaffold, but sometimes the construct would deform, or even disintegrate, under the applied stresses.
However, Professor El Haj and colleagues demonstrated that they could not only seed the scaffolds with bone marrow stem cells that had been tagged with the magnetic nanoparticles, but then use magnetic forces, instead of crushing mechanical loads, to stimulate tissue growth. The researchers went on to activate cells and grow tissue in a range of scaffold biomaterials, demonstrating that the technology could be applied to many soft, biocompatible and biodegradable materials.
Using a bioreactor to successfully grow tissue from stem cells has been a massive leap forward for cell therapy, but researchers around the world also want to grow tissue in vivo, that is, within the body. With this in mind, El Haj and colleagues have grown tissue in vivo, in mice, using a slightly different technique.
They first tagged bone marrow stem cells with magnetic nanoparticles and then encapsulated the tagged cells with alginate-chitosan microspheres, which served as the scaffold for tissue growth. They embedded the capsules in the backs of several mice, and then exposed them to magnetic fields. In doing so, they were able to trigger stem cell differentiation and bone tissue growth remotely within the mice. The implications are profound.
If this process can be repeated in humans, the researchers may have found a way to treat arthritis and a range of other diseases without the need for major surgery, artificial, or even donor organs. And excitingly, additional research is bringing El Haj and colleagues even closer to this goal. A new technique is under development that could one day allow doctors to repair damaged bones and joints anywhere in the body, simply by injecting stem cells into a patient’s arm (see Figure2:Clinical vision).
Working with colleagues from the Vienna-based Ludwig Boltzmann Institute, one of the leading centres in Europe for studying wound repair, researchers have managed to localise stem cells to a target site in mice using magnets from outside their bodies. Stem cells taken from human fat, encapsulated and tagged with magnetic nanoparticles, were injected into the tail veins of mice. Using an in vivofluorescent imaging system, researchers then guided the capsules to a wound within the skin using a rare earth magnet. They are now working on how to ‘hold’ the capsules in place and switch the stem cells into action to regenerate tissue with plans for pre clinical trials underway, althoughin vitroresults also look promising.
Using laboratory-based circulation systems that mimic the in vivoenvironment, the researchers have established the key principles behind stem cell delivery using magnetic nanoparticles. Given their progress, they hope to move to human trials within the next five years.

Figure 2. Clinical vision: after extracting and tagging the stem cells, the tagged cell could be injected into a repair site in the knee, with a magnetic cuff being placed over the knee to ‘hold’ the cells at the site and stimulate cell differentiation to encourage new tissue growth
Looking further
Beyond orthopaedics, magnetic nanoparticles could be used in a host of other healthcare applications. Dynamic drug screening for the pharmaceutical industry is one example. During early-stage drug screening, laboratory cells could be tagged and activated to mimic the complex cell behaviours that take place in the body.
Local anaesthesia without drugs is another potential application. Ion channel receptors on cells are involved in anaesthesia; could it be possible to use magnetic nanoparticles to open these receptors in the same way as an anaesthetic? If so, the nanoparticles could be moved to any part of the body and provide much more localised anaesthesia than any drug could.
And a recent development will only speed progress. In early October 2011, Arthritis Research UK launched a £6 million tissue engineering centre that promises to help the nation’s Eightmillion osteoarthritis sufferers. The venture brings together clinicians, engineers and biologists from the Universities of Keele, Aberdeen, Newcastle and York, with the hope of using stem cells to regenerate the damaged bone and cartilage in osteoarthritic patients.
Much of the research carried out at Keele University will be instrumental to this aim: indeed, the stem cell injection for early-stage osteoarthritis could be available in as little as five years. If this proves successful, a ‘one-stop’ day procedure could be possible that could delay the need for joint replacement surgery.
However, many of the new centre’s researchers are looking even further forward. Is it possible to ‘switch on’ stem cells already present in patients’ joints? And while today’s stem cell treatments use the patient’s own cells, future therapies could use cells from anyone. UK and US trials are already underway using both embryonic and engineered donor cells. Could a bank of universal donor cells, that can be grown and processed for delivery to a patient’s bedside, be next?
Keep up-to-date with Ingenia for free
SubscribeOther content from Ingenia
Quick read

- Environment & sustainability
- Opinion
A young engineer’s perspective on the good, the bad and the ugly of COP27

- Environment & sustainability
- Issue 95
How do we pay for net zero technologies?
Quick read

- Transport
- Mechanical
- How I got here
Electrifying trains and STEMAZING outreach

- Civil & structural
- Environment & sustainability
- Issue 95