Making Light Work
Copyright Optical Trapping Group University of St Andrews
At the micrometer to nanometer scale, powerful light sources have unique properties that are now being harnessed by researchers to give new insights into biological mechanisms, chemical reactions and all-optical circuits. At the heart of these breakthroughs is the harnessing of the mechanical attraction of light for tiny objects, seen most obviously in ‘optical tweezers’. Professors Will Stewart, James Wilkinson and Kishan Dholakia write about the progress being made with optical manipulation tools.
At everyday scales, light can provide warmth, it can illuminate, and it can even be used to slice metal – powerful laser beams are now routinely used to cut steel for cars. But light is not known for pushing things around. At the nanometer to micrometer scale the rules are different. Light beams can lift and manipulate tiny objects without touching them and with extraordinary precision and gentleness, an effect now being harnessed to carry out engineering on single cells and micromechanical circuits, and even single molecules.
General experience tells us that light does not have the power to move objects around. The heat that light creates perhaps does, but not the light itself. But as we enter the world of micron-sized cells and nanometer-scale structures, this changes. At this scale, the force of light can become stronger than gravity and other, normally more significant, forces. It can be used to move and place delicate cells without touching them; trap slippery objects for analysis; propel particles along microfluidic networks towards a reaction with other compounds (see Lab-on-a-chip, Ingenia39); switch ultrafast optical circuits; and manipulate molecular motors in biology.
How it works
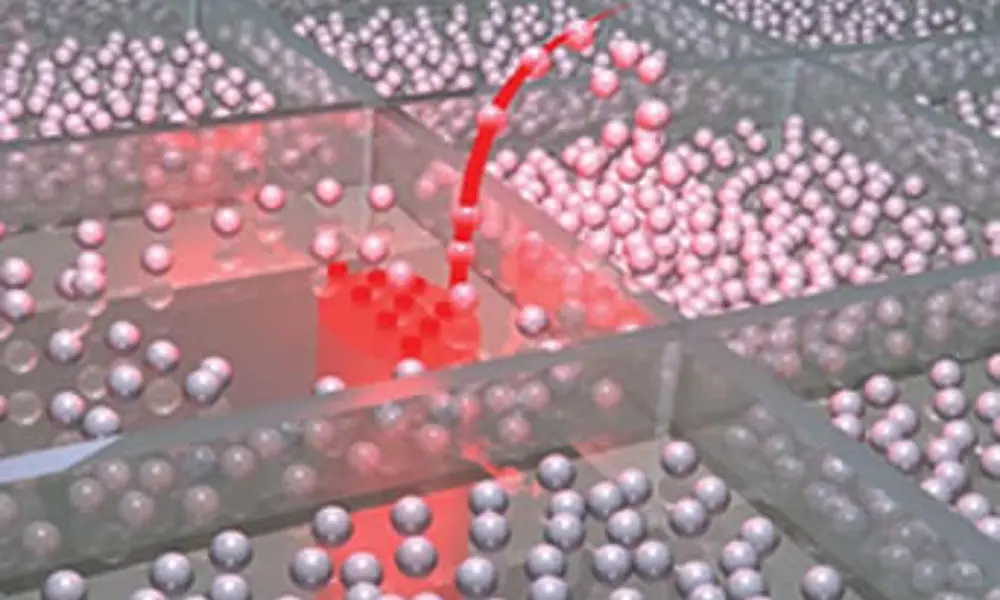
Though the photons that make up light have no mass, photons may exert a force because they travel at the speed of light. Einstein’s theory of relativity allows them to carry both momentum and energy, and this means they can produce forces. And just as a plastic duck can be held in a stream of falling water, so a tiny particle can be held in a beam of light. The force of light pulls denser particles into regions where the light is brightest. So if the light beam is focused to a small point, a particle can be held at that point, even being lifted towards the light source because the intensity falls as the beam spreads out beyond the focus.
A particle is trapped in the focused region because, as it moves away from the point of focus, it ‘swings’ the beam sideways in the same direction and the reaction force on the particle from the momentum of the deflected beam will then tend to push the particle back (see diagram alongside). Returning to the duck and water analogy, the major advantage a beam of light has over a stream of water is that it can be focused like this. This means that, as well as controlling the position of a particle in an x-y direction (sideways), light can also control the position of a particle in the z direction (up-down).
The scale effect is a vital part of this. Small particles can be trapped because their weight can be similar to the trapping forces for relatively low-power beams. If the whole process is scaled up and use a focused ‘tractor’ beam designed to be big enough to lift a large object, then the power of the beam becomes so high that the object would be immediately destroyed by heating.
Optical Tweezers
This might all seem a bit far-fetched but much of this technology is already being used and in some cases is commercially available. For example, ‘optical tweezers’ are devices that let biologists manipulate cells without damaging them and they are available as an add-on to many modern microscopes. Around half a dozen companies now sell ‘traps’ based on optical tweezers. These range from basic systems to move single objects around, to multiple trap arrays in two or three dimensions, using holographic or fast beam switching technology to make arrays of optical bright spots that can move large sets of objects in synchrony.
Some of these systems also include optical traps whose pulling strength is carefully calibrated. These can then be used to weigh objects by seeing how much force is needed to lift them and to examine naturally moving biological motors to measure the tiny forces they produce. These vital molecular motors are the engines that run cells, including humans’, and produce mechanical forces from chemical energy. Although we are more familiar with biological ‘linear motors’ at the cellular level they can also be rotational like an electric motor. In either case the pico-Newton (pN) forces are very hard to measure, particularly at cellular scales, hence the significance of the non-contact optical trap in measuring them by gently pulling against the cells.
The same techniques can even be applied to manipulating single molecules, though this is most easily done by anchoring the molecules to inert glass beads a micron or so in diameter. Researchers have used this to watch the DNA processes of life itself.
Optical tweezers can also be used in integrated microsystems to propel particles with molecules attached along microfluidic networks for reaction and separation. Using optical waveguide technology developed initially for the optical telecoms industry, microfluidic channels or ‘lab-on-a-chip’ technology can be used to analyse a variety of types of sample in a fast and effective way.
Waveguide technologies
Optical waveguides confine most of the light within themselves but also exhibit an exponentially decaying ‘evanescent’ field away from the waveguide surface into a surrounding medium such as blood plasma. The resultant intensity gradient attracts nano and micro particles to the waveguide track and the scattering and absorption forces propel the particles along the track at a rate dependent on their properties. This powerful technique has been used by researchers to trap and propel red blood cells, gold nanoparticles and, very recently, dielectric nanoparticles and strands of DNA.
Researchers at the Institute of Scientific Instruments in the Czech Republic have constructed a standing wave in an evanescent field which acts as an optical conveyor belt by establishing a string of 3D traps which can be made to move along the guide by progressively shifting the optical phase.
The evanescent field that extends beyond an optical waveguide can move surrounding particles but it can also attract and move another nearby waveguide and the light can thus be switched between the guides.
Again, intuitive expectation may be misleading at these small scales and these opto-mechanical switches are not only robust but approach the speed of similar-scale electronic transistor switches. Since optical switching circuits route a growing proportion of the vast amounts of data carried over today’s (photonic fibre) internet, this is very important. It may also help to address a key problem with the electronic versions of such switches: they use large and growing amounts of electric power, most of which is wasted in converting information between electronic and optical forms just because the electronic form is easier to switch.
Optical waveguide devices that are switched by forces from the light travelling through them have been demonstrated by various research groups around the world, including the University of Southern California, the California Institute of Technology, and Yale University, Connecticut.
An early example consisted of two parallel waveguides in the configuration of a directional coupler (a device used in electronics to allow non-disruptive sampling of a wave transmission). Similar gradient forces to those which trap particles in the optical tweezers and the conveyor belt described above cause the two waveguides to be attracted to each other or repelled, depending on the phase of the light travelling in them. This then causes the waveguides, which are free to flex in the coupling region, to move closer or to separate. As a directional coupler operates by switching light from one waveguide to the other depending upon waveguide separation, this optically driven movement can cause the light to switch between the guides (see Optical Switching).
Further trap applications
With so many different uses, optical traps, or optical tweezers, have a huge number of potential applications, many of which have yet to be thought of. In addition, it would be valuable to monitor multiple beads in real time and have multi-point position and force analysis. This will assist the next generation of cell biological studies where traps can probe multiple points on a membrane simultaneously.
Optical tweezers enable us to do things now that we never thought possible: watch the process of molecules interacting or proteins unfolding, switch optical circuits or manipulate chemical reactions. Optical tweezers are rapidly becoming essential for biotechnology and photonic research and their usefulness will increase over the coming years.
Optical Switching
Modulation of light by light was demonstrated with great elegance by researchers at the California Institute of Technology by using a planar microdisk resonator (in which light is trapped like whispers in the dome of St Paul’s Cathedral) coupled to an optical fibre nanowire. With less than a milliwatt of optical control power, over 99% of the light was switched. Switching was achieved by moving the nanowire up to 1 micron, entirely with optical forces. A monolithic integrated version of this device should offer GHz operation.
Researchers at Yale University in the United States have demonstrated a different fully CMOS-compatible monolithic approach, where light in a cantilever nanowire waveguide tunnelled into the underlying substrate via a 0.3 micron air gap. The gradient force was used to attract the cantilever towards the substrate, modulating both the strength of tunnelling of light into the substrate, and the speed of the light in the waveguide. In this case the mechanical system was designed to resonate near 10MHz, but on-chip optical resonators have been demonstrated with optically-excited mechanical resonances above 1 GHz, showing promise for high-speed opto-mechanical systems.
Optical tweezers for protein folding measurements
Optical tweezers enable us to study the function of individual cells and molecules to shed light on complex life processes at the molecular level. Optical tweezers can be used in biological fluids so that molecules can be studied in their natural environment. A protein molecule consists of a well-defined sequence of amino acids that folds up into a 3D shape, defining the function it performs in controlling biological functions.
The binding of small molecules can dramatically change the conformation of a protein and thus its function, so that understanding folding is key to understanding life. Optical tweezers can exert small forces on molecules such as proteins, thereby enabling study of the dynamics of protein folding.
In one elegant example in the US, researchers at the University of California, Berkeley, attached two micron-sized polystyrene beads to an individual E coli ribonuclease H molecule at different positions, as ‘molecular handles’, via two double-stranded DNA molecules. One bead was anchored and the other was trapped using optical tweezers. The protein was stretched and relaxed repeatedly by moving one bead, causing the protein to unfold and refold.
The forces required to cause intermediate and complete unfolding were measured in the range of 0-30 pico-Newtons, by observing the effect of force-driven displacement of the trapped bead within the trap upon the transmitted light. This technique has been applied to many individual molecules providing important information on protein function.
BIOGRAPHIES – Professors Kishan Dholakia FRSE, Will Stewart FREng and James Wilkinson
Professor Kishan Dholakia FRSE has worked on various aspects of trapping and biophotonics for the last 15 years. His work has focused on novel light fields for trapping and sorting as well as using light for cell transfection. He works at the School of Physics and Astronomy, the University of St Andrews.
Professor Will Stewart FREng is an expert on photonics, communications and electromagnetics. He is on Ofcom’s Spectrum Advisory Board, ECOC management committee, chairs the IET’s Communications Sector panel and many others. He is a Visiting Professor at University College London and at the Optoelectronics Research Centre at the University of Southampton.
Professor James Wilkinson studies the application of optical waveguide techniques in communications, medical and environmental sensing and lasers in the Optoelectronics Research Centre at the University of Southampton. His present activities focus on optical manipulation and detection of micro- and nano-particles in microfluidic systems and optical nanowire circuits.
Keep up-to-date with Ingenia for free
SubscribeOther content from Ingenia
Quick read

- Environment & sustainability
- Opinion
A young engineer’s perspective on the good, the bad and the ugly of COP27

- Environment & sustainability
- Issue 95
How do we pay for net zero technologies?
Quick read

- Transport
- Mechanical
- How I got here
Electrifying trains and STEMAZING outreach

- Civil & structural
- Environment & sustainability
- Issue 95