
Replacing the batteries
Faraday battery challenge
🔋 Advancing battery development in the automotive industry and beyond
The Faraday Battery Challenge is a government investment in research and innovation projects and new facilities to scale up and advance the production, use and recycling of batteries. It aims to lower the UK’s carbon emissions and air pollution and create new opportunities and industries.
The project currently focuses on the automotive industry, but its work will also help advance battery development for other applications. The investment of up to £246 million will develop batteries that are cost-effective, high-quality, durable, safe, low-weight, and recyclable.
The investment covers three areas: collaborative research and development projects, which have so far included improving battery lifespan and range as well as the reuse, remanufacture and recycling of batteries at their end-of-life; an £80 million investment to create the UK Battery Industrialisation Centre developed by Coventry and Warwickshire Local Enterprise Partnership, WMG at the University of Warwick and Coventry City Council, which allows companies to quickly develop their capabilities to manufacture batteries and get them to market, scale up and go global; and the Faraday Institution, where academics are working with industry on research, training and analysis into batteries, and helping develop technologies for the future.
Demand for action on climate change has prompted the government to declare a climate emergency and to pledge that the UK will eliminate the country’s greenhouse gas emissions by 2050 – an ambition that poses an array of engineering challenges.
With transport making up around 20% of the world’s energy consumption, electric vehicles will be essential to achieving net zero. Transport is the largest sector for greenhouse gas emissions in the UK, with road transport accounting for more than 90% of the domestic emissions. In 2018, electric vehicles were just 1.1% of the 2.37 million new cars sold in the UK; 6.2% including petrol–electric hybrids. However, in 2019 sales jumped by 60% with nearly 12,000 sold in the first half of the year and more than 64,000 hybrids and plug-in hybrids sold in the same period. Manufacturing these vehicles requires innovation and engineers are continuously developing new approaches to improve batteries’ performance, characterisation and use.
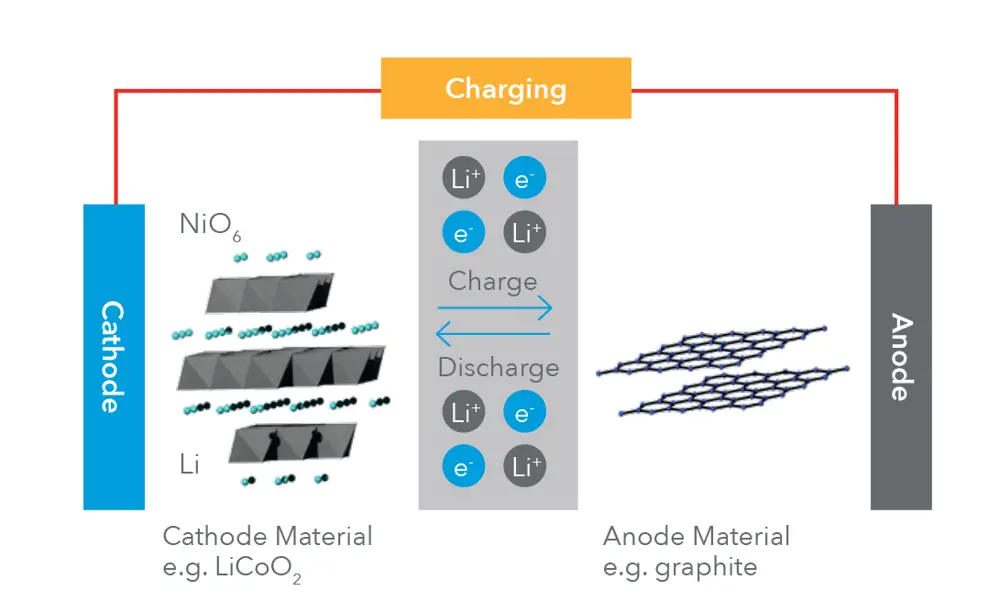
The anode, cathode, separator, external circuit, and flow of Li-ions and electrons in a typical Li-ion battery © WMG
The Faraday Battery Challenge is an important part of the UK’s R&D into batteries for electric vehicles. Supported through the Industrial Challenge Strategy Fund, the Faraday Battery Challenge, with a budget of £246 million over four years, covers R&D in all aspects of battery technology (see ‘Faraday Battery Challenge’). The £78 million Faraday Institution focuses on fundamental research into battery technologies.
In an electric vehicle, the battery is far more than an energy reservoir. Its array of sensors and electronic systems define almost everything we care about in a car and the 600-kilogram mass of cells underneath the seats is at the heart of the vehicle’s design. Battery assembly accounts for half of the cost of building a car, and its capacity governs the driving range. Pressure is on to reduce the cost, and weight, of batteries and to increase their storage capacity.
Most current electric vehicles use a range of hundreds of individually instrumented lithium-ion (Li-ion) cells, the batteries with the highest energy density in commercial use (see ’Lithium-ion batteries: a brief introduction’). These have cathodes that contain cobalt, an expensive raw material, some of which comes from unethical sources. Some suppliers, including Panasonic, the world’s largest battery maker, have set themselves the goal of removing cobalt from their products.
Lithium ion batteries: A brief introduction
⚡ The properties that led to lithium becoming a preferred power source
There are many types of lithium-ion (Li-ion) battery, but all are made up of cells: a positive electrode, a negative electrode and an electrolyte. When the battery is charging, lithium ions from the positive electrode move through the electrolyte to the negative electrode. When the battery supplies power, lithium ions move back to the positive electrode. In both cases, electrons flow in the opposite direction to the ions, around the external circuit.
Lithium is an extremely light metal with significant electrochemical potential and energy density for its weight, which made it a subject for battery research from the 1910s to the 1970s when the first non-rechargeable lithium batteries were produced. Unfortunately, lithium’s instability made it unsuitable for recharging as dendrites would form and penetrate the separating barrier between the electrodes, causing an electrical short and rapid increase in temperature. As a result, in the late 1970s and early 1980s, researchers investigated compounds such as lithium cobalt oxide and carbon for the electrodes, leading to the development of today’s Li-ion batteries. These progressive advances in technology led to John Goodenough, M. Stanley Whittingham and Akira Yoshino winning the 2019 Nobel Prize for chemistry.
Modern Li-ion batteries offer a high cell voltage, are lightweight and can be recharged repeatedly without losing as much capacity as older batteries. This makes them a preferred power source for energy hungry and mobile applications from smartphones to electric cars.
Finding alternative battery technologies
Engineers are researching various alternative battery technologies. One approach is to use so-called solid-state batteries, with solid lithium metal electrodes and solid electrolytes, rather than the flammable organic liquid electrolytes in Li-ion batteries. Mainly used in medical technologies, commercial solid-state batteries could increase a car’s driving range, reduce weight and address safety concerns such as flammability and leakage. However, solid-state batteries can fail after repeated charging and discharging at the high current density needed for many consumer applications, preventing their wider adoption.
This failure has been explored by the SOLBALT project at the University of Oxford, which is supported by the Faraday Institution. It has discovered that voids can form and accumulate during charging when current density removes lithium from the interface between the lithium metal and the ceramic electrolyte faster than it can be replenished during discharge. This increases the local current density, leading to dendrite formation (fingers of molten metal that form during battery charging) and battery failure. To prevent dendrite formation, cells need to be charged and discharged below the critical current density at which voids start to form.
As well as these newer technologies, researchers are investigating new uses for older battery technologies. Sodium-ion batteries were developed at the same time as Li-ion batteries and work in the same way, with sodium compounds instead of lithium compounds. However, sodium ions are larger than lithium ions, so the batteries have naturally lower energy density, which limits commercial interest.

A researcher at WMG works on new battery pouch cell technology © ERA commercial photography. Photo by Alex Wilkinson Media
One advantage of sodium-ion batteries is that they operate at lower voltages than Li-ion. This means that aluminium can be used in place of copper for the anode current collector without it degrading electrochemically at low states of charge. This lower voltage is also why the electrolytes are cheaper. This makes sodium-ion batteries a viable alternative to lead-acid batteries in scooters, e-bikes and even e-rickshaws, where energy density is less important than cost. Faradion, established in 2011 in Sheffield as the world’s first commercial sodium-ion battery maker, demonstrated the world’s first sodium-ion e-bike in 2015. Its current prototype
cells have an energy density of 140 watt-hours per kilogram (Wh/kg), more than three times the capacity of lead-acid batteries. These batteries can also be a drop-in replacement for lead-acid batteries, offering greater range and faster charging at a similar cost. As battery charging networks develop, sodium-ion batteries could become a solution for passenger vehicles: recent research has shown that sodium-ion batteries can be fully charged in just 20 minutes, which would allow brief ‘refuelling’ stops and longer operation time.
Lithium-sulphur (Li-S) batteries are also becoming attractive. The theoretical energy density of a Li-S cell is 2,510 Wh/kg, far higher than the 600 Wh/kg or so for Li-ion batteries. Alongside the energy advantages of Li-S cells, sulphur is easy to source and is non-toxic, making it an environmentally friendly alternative to Li-ion cathodes. However, these advantages also present challenges. Li-S cells have a relatively short cycle life. This is in part because the lithium anode reacts with organic solvents, which, over time, can cause a decline in charging efficiency and a reduction in discharge capacity. Much of the research on Li-S batteries has focused on protecting the anode, which would improve their cycle life significantly.
Li-S has a lower volumetric energy storage density than Li-ion, so Li-S cells will be lighter, but they are also likely to be larger. While this might rule them out for some commercial uses such as cars, they may be more suitable for high altitude and long-range aerospace applications. UK-based Oxis Energy has worked with Bye Aerospace on electrifying piston and turboprop aircraft for regional flight transportation. The company has also investigated possible marine applications. The company, which develops Li-S batteries for a range of industries, is working with the National Oceanography Centre to develop a Li-S battery that can power autonomous marine vehicles at depths of more than 6,000 metres. Through the Revolutionary Electric Vehicle Battery project, funded by InnovateUK, Oxis also worked with Imperial College London, Cranfield University and engineering company Ricardo to develop a 400 Wh/kg cell for a battery electric vehicle, with plans to produce a 500 Wh/kg cell later this year and ambitions to reach 600 Wh/kg.
Recycling
♻️ Recapturing valuable metals, using robots and the EU’s Battery Directive
Batteries from electric vehicles (EVs) have a lifespan of around ten years before they need replacing. An EV battery reaches the end of its automotive life when its capacity falls to 80% of a new battery.
The Global Battery Alliance, a World Economic Forum initiative to accelerate and scale up action towards an innovative and sustainable battery supply chain, estimates that there will be 11 million tonnes of spent Li-ion batteries globally by 2030. The good news is that various recycling methods are already in commercial use and could deal with the pending landslide of used car batteries.
The batteries in most EVs use Li-ion cells, and older hybrids use nickel metal hydride. They rely on metals such as lithium, copper, nickel, manganese, and cobalt to provide energy-dense cells that are relatively small and light. The cell itself – complete with these metals and a highly flammable electrolyte – makes up around 60% of the battery. The casing accounts for 30% while the rest of the package consists of wiring, cooling tubes, busbars, screws, adhesive and embedded electronics, an important part of the control system that monitors the health of each cell and manages the energy flow. Over 90% of the cell can technically be recovered. However, recycling is a commercial activity that involves the reuse of recovered materials. Many companies will only recycle parts that give them a financial return.
Li-ion batteries are covered by the EU’s Battery Directive, which stipulates that at least 50% of the entire battery must be recycled. Battery packs are dismantled manually. The plastics and wiring that make up the bulk of the pack around the cell can be recycled along with other similar plastics. This is straightforward and achievable.
The main difficulty is that the valuable metals are embedded in electrodes and surrounded by volatile electrolyte. The electrolyte is a solution of organic carbonates and a conducting salt that is flammable, explosive and highly toxic. Any recycling process must deal with these hazards before the rest of the cell can be recycled. This electrolyte makes battery recycling more hazardous than conventional recycling of plastic and metal. In future, advanced robotics could aid the recovery of battery content, reducing potential harm to human operatives.
To this end, the National Centre for Nuclear Robotics is examining how to build robots to operate in extreme environments. It is working with the Faraday Institution to produce robots using artificial intelligence to remove batteries from vehicles for final recycling. The Faraday Institution’s ReLiB project on recycling and reuse of Li-ion batteries sets out to improve the recovery, re-use and recycling of batteries, with the ultimate aim of retaining almost 100% of the battery components within the automotive sector.
The first step in improving the efficiency of battery recycling is learning how to accurately assess the state of a battery before it is removed. In this way, the batteries can be safely transported to the relevant recycling or reuse facility. Some companies use high temperature processing to destroy the electrolyte and then treat the toxic gases generated. This approach, called pyrometallurgy, involves processing batteries in a furnace. Other recyclers use specialist technologies, such as shredders, to cut open cells in an inert atmosphere and then treat or capture the electrolyte and electrode materials. Once this is done, the remaining battery components can be recycled in the usual way.
The cathodes in batteries contain lithium and cobalt, valuable metals that can be extracted for reuse in new batteries. In the process the metals lose none of their electrochemical properties, so a battery made with recycled metal is just as efficient as one made with freshly mined metals. Recapturing these metals is the most important aspect of battery recycling. The metals, particularly cobalt, are expensive and mining them is resource-heavy and environmentally damaging.
The ability to recycle batteries efficiently makes economic, environmental and moral sense, which is why many manufacturers are beginning to focus on the idea of ‘circular economy’ battery manufacture and reuse.
Copy written by Vicky Parrott, freelance journalist
How Formula E car racing drove the development of batteries
The variety of battery technologies available and in development suggests that the future of electric vehicles doesn’t stop with cars. There is the potential to reengineer aircraft, commercial vehicles and public transport, and to develop batteries to meet each vehicle’s needs.
Formula E car racing has been a high-profile success story for battery development. Williams Advanced Engineering, a part of the Formula 1 car company, developed the first battery packs for Formula E. When first launched in 2013, Formula E cars could not complete a race, meaning that drivers had to switch cars mid-event to go the distance. Battery technology progress means that cars can now complete the race. The latest Formula E batteries, developed by Atieva, a part of Lucid Motors, have cells specifically chosen for their race performance, built in a unique trapezium design to fit maximum power inside the car. They contain software that allows the battery pack to perform for the duration of the racing season with no significant degradation.
The emphasis on performance and improved power in racing cars has also helped innovation in aerospace, where the goal is to develop all-electric fixed-wing commercial flights. Rolls-Royce’s research programme Accelerating the Electrification of Flight aims to develop the fastest all-electric plane in history to break the current 466 kph world record. The single-seater plane, powered by what Rolls-Royce dubs the “the world’s most energy-dense flying battery pack”, would have enough energy to fly from London to Paris. Taking inspiration from the successes of Formula E batteries in reducing weight, the team used lightweight materials to cut the plane’s packaging-to-battery cell weight ratio in half when compared to an electric car, as well as building in a bespoke liquid cooling system to regulate the heat of the battery cells. The aircraft has three 72 kWh batteries, combining 18,000 Li-ion battery cells, packaged for maximum lightness.
Electrifying transport in the pursuit of net zero
Maximising energy density and minimising weight are essential to high performance road and passenger aerospace vehicles, but in other fields batteries need different qualities that can bring added benefits. For example, when JCB began designing an all-electric mini-excavator it became clear that the vehicle would be ideal for work indoors, in tunnels or even underground, as there would be no need to extract toxic combustion fumes. It produces just a fifth of the noise of a traditional diesel engine model, an advantage for work in busy cities.
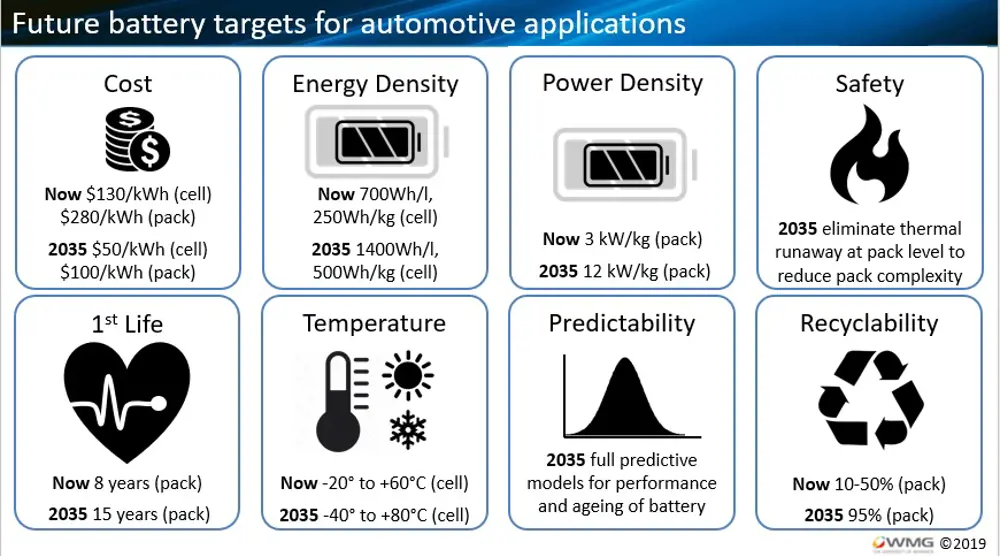
Future battery targets for automotive applications
Electrifying transport in pursuit of net zero requires more than just finding more efficient and economical ways to store electricity. Engineers also need to find ways to make electric vehicles fit into our lives. While new technologies and applications are important, existing technology needs improvements, such as quicker charging. The ability to fast-charge car batteries is essential in reducing the fear that a car’s batteries will run flat in the middle of a journey. However, current fast-charging technology can produce triple the heat energy of slower charging. Li-ion batteries operate best inside a relatively narrow temperature range, roughly 15°C to 35°C: too cold and they charge too slowly, while excessive heat can degrade performance and can accelerate battery ageing. Good thermal management is the key to better battery performance, more range on a single charge and a longer cell life.
In another programme funded as a part of the Faraday Challenge, research is under way to cool batteries in use. The I-Co Bat research programme is developing a battery pack concept that uses a biodegradable cooling fluid, MIVOLT, to immersion-cool the battery. MIVOLT is developed from dielectric fluids currently used to cool and insulate transformers in wind turbines and high-speed trains. The batteries are immersed in coolant to reduce heat, improve charging time, and lengthen battery life. A research collaboration between WMG at the University of Warwick, Manchester-based M&I Materials, which has more than 40 years’ experience in manufacturing products for electrical insulation, and electric vehicle battery pack and management specialists at Ricardo, is aiming to validate and commercialise this new form of battery cooling.
Whether developing sustainable battery technologies, finding new uses for established battery types, improving the performance of existing batteries or giving new life to old batteries, UK engineers are transforming the way we power our world. In the near future, batteries will power planes, ships, construction vehicles, homes and cars, helping deliver a zero-carbon economy and a cleaner world.
***
This article has been adapted from "Replacing the batteries", which originally appeared in the print edition of Ingenia 83 (June 2020).
Keep up-to-date with Ingenia for free
SubscribeRelated content
Electricals & electronics
Accelerometers
Used in earthquake measurements, laptops, planes and even in stargazing apps, today’s accelerometers are much smaller than when they were first developed in 1927. Find out how they detect movement and vibration.

How to maximise loudspeaker quality
Ingenia asked Dr Jack Oclee-Brown, Head of Acoustics at KEF Audio, to outline the considerations that audio engineers need to make when developing high-quality speakers.

Cable fault locator
The winner of the Institute of Engineering and Technology’s 2014 Innovation Award was EA Technology’s CableSnifferTM, which uses a probe and chemical sensing technology to identify faults, saving energy companies millions of pounds each year.

High speed evolution
In December 2010, Eurostar International Ltd awarded a contract for 10 new high speed trains to Siemens. The company has used a system developed over decades to maximise the performance and passenger-carrying ability of its 320km/h trains.
Other content from Ingenia
Quick read

- Environment & sustainability
- Opinion
A young engineer’s perspective on the good, the bad and the ugly of COP27

- Environment & sustainability
- Issue 95
How do we pay for net zero technologies?
Quick read

- Transport
- Mechanical
- How I got here
Electrifying trains and STEMAZING outreach

- Civil & structural
- Environment & sustainability
- Issue 95