
Responding to a global pandemic with ventilators and PPE
An increase in the rate of COVID-19 infections led to the UK’s engineering community gearing up to meet urgent social needs by innovating, repurposing and ramping up production. Since March, engineers in research and business have worked intensively to deliver solutions, often improvising at pace in close consultation with medical professionals.
At the outset of the pandemic, the NHS had just over 8,000 ventilators to save the lives of severely ill patients who developed breathing problems. An early projection suggested that more than 60,000 ventilators could be needed, and the UK’s capacity for manufacturing ventilators was estimated at just 2,000 units per year. The government announced plans to source 30,000 new machines by increasing production of existing designs, importing thousands more, and commissioning fresh models. However, this projection was later revised to 18,000, meaning 10,000 more machines were needed.
As part of this, the government’s Ventilator Challenge tasked businesses to make safe, easy-to-use ventilators, at scale, according to its specification for rapidly manufactured ventilator systems. Of 15 projects shortlisted by the government after about 5,000 initial offers, two of the most successful looked at how to reconfigure and accelerate production of existing models. Both came from the Ventilator Challenge UK (VCUK) consortium of industrial, technology and engineering firms, including Formula 1 teams, Airbus, Siemens, and Rolls-Royce. This lent manufacturing muscle to scale up production of a reconfiguration of Penlon’s Prima – used in operating theatres – and Smiths Medical’s ParaPAC, a mechanical system.
VCUK approached anaesthesia equipment manufacturer Penlon, which believed it could meet the specification by reconfiguring sub-modules into a more compact and affordable solution that was easier to manufacture. The existing production rate was between six and ten units a week, but the consortium needed to get closer to 3,000 a week. Production lines completing 250 different activities spanned from North Wales to Dagenham, Hampshire and Oxfordshire, involving 2,200 people.
We’ve learned that if you move very fast and fail very quickly, you don’t actually set yourselves back. If we harness that, we will make much greater progress going forward
One engineering challenge was to secure the supply of strategic components. Each of Penlon’s reconfigured machines has about 700 parts, many obtained from other countries and some made using now-obsolete processes. “We had to reinstate production lines in Israel in order to produce printed circuit boards,” said Dr Graham Hoare OBE FREng, Executive Director and Chair of Ford of Britain, who led this project on behalf of VCUK, at an online Q&A event organised by the Royal Academy of Engineering*. Another challenge was to train thousands of new workers. “We had to develop an entire remote training strategy,” said Dick Elsy CBE, Chief Executive of the High Value Manufacturing Catapult and VCUK’s Chair. “We used Microsoft HoloLens to train people in the production facility [from other locations] … and had well over 100 HoloLenses in use. It was remarkable how people responded and made the tools work.”
Dr Hoare described how pressures that necessitated speedy execution helped to engender rapid new learning and collaboration. One example came from Siemens, which involved its apprentices to address a complex needle adjustment. This team created a digital twin of the manufacturing facility and the parts, solved the problem in the digital world, 3D printed the solution, tested it in production, and found a way of automating it in four days. Dr Hoare reflected: “That speed of execution is something we will take forward. We’ve learned that if you move very fast and fail very quickly, you don’t actually set yourselves back. If we harness that, we will make much greater progress going forward.”
In mid-April, the consortium’s Penlon Prima ES02 gained approval from the Medicines and Healthcare products Regulatory Agency (MHRA) and the government ordered thousands of units. “We were very focused on getting everything ready,” said Elsy. “It helped that Penlon is used to dealing with the regulator, but we achieved regulatory approval in 21 days – it normally takes six to eight months.”
How mechanical ventilation works
🌬️ How CPAP ventilators can help COVID-19 patients
The ordinary function of breathing involves an intricate and complex process. In normal breathing, when the diaphragm muscles tighten it expands the lungs, reducing the pressure in them and drawing air in. On relaxation, pressure in the lungs rises again forcing air out.
COVID-19 infections can make normal breathing very difficult. Inflammation can irritate the airway lining and alveoli: these tiny air sacs become clogged, pouring fluid and inflammatory cells into the lungs, and losing the ability to transfer oxygen to the capillaries, or dispel carbon dioxide. When a patient develops acute respiratory distress syndrome (ARDS), fluid builds up in the sacs and oxygen levels plummet.
As an illness unfolds, a patient may require mechanical ventilation, either non-invasive, invasive or both. This involves pushing oxygen-rich airflow into the lungs to help the patient breathe, which also frees up energy for the body to focus on fighting infection.
The first ventilators, such as the ‘iron lung’, came into use nearly a century ago and typically used negative pressure: making the chest expand and contract to suck air into the lungs. These fit over the chest and pull air in. These days, positive-pressure ventilators fit over the face and help patients breathe by pushing air into the lungs: blowing and stopping in cycles, ventilators enable patients to take in oxygen and dispel carbon dioxide.
There are three forms of non-invasive ventilation using positive airway pressure: autotitrating (APAP), bilevel (BiPAP), and continuous (CPAP), the latter of which has been used successfully with many COVID-19 patients. CPAP helps patients who can breathe by themselves, and involves channelling oxygen through a facemask to keep airways unobstructed. It works by applying mild pressure continuously throughout the breathing cycle.
A CPAP machine delivers a constant flow of pressurised oxygen-rich air to create a cushion along the upper airway: this helps to hold open the alveoli, but it does so continuously rather than only at the end of the exhalation. Patients remain conscious and may be on CPAP for up to four days.
Invasive ventilation, sometimes used for several weeks when someone is very sick, involves inserting a tube into the airway. The ventilation unit also has a humidifier to match air to body temperature and add moisture and can hold a constant amount of low pressure to keep air sacs in the lungs from collapsing. The tube allows mucous to be sucked away from the windpipe.
Today’s ventilators are highly sophisticated, giving healthcare professionals more ability to monitor and tailor treatment to individuals.
Creating innovative designs for mechanical ventilators
Many of the engineers who joined the race to produce mechanical ventilators started from scratch. OxVent is a simple and scalable design put together in less than a fortnight, and conceived as an open-source, not-for profit project. A team of University of Oxford academics created a working prototype in four days, with help from technicians at the Institute of Biomedical Engineering. They worked to identify a mechanism requiring minimal moving parts, aiming to overlap as little as possible with the conventional ventilator supply chain. The simple design has a feedback loop to control flow delivered to the patient, but does not monitor the patient’s physiological signals.
Testing and validation were a critical part of the rapid innovation process. Ventilators have a straightforward core function (see ‘How mechanical ventilation works’) but to sustain life reliably, a design must undergo rigorous and lengthy testing. Like the design, testing had to be developed from scratch, but the team found there was a run on testing equipment. “We had a lucky break: a colleague got us a measurement system to look at the characteristics of flow and volumes delivered in lung models, and that ended up being critical for our test methodologies,” says Professor Tim Denison, Royal Academy of Engineering (RAEng) Chair in Emerging Technologies.
With the UK’s need for ventilators no longer as critical, the government has not taken OxVent forward, but its team is now adapting the work to evolving global needs and aims to gain FDA Emergency Use Authorisation. It is also pursuing regulatory approval in countries where COVID-19 cases are increasing, and envisages OxVent’s rapid manufacturability will see it come into its own.
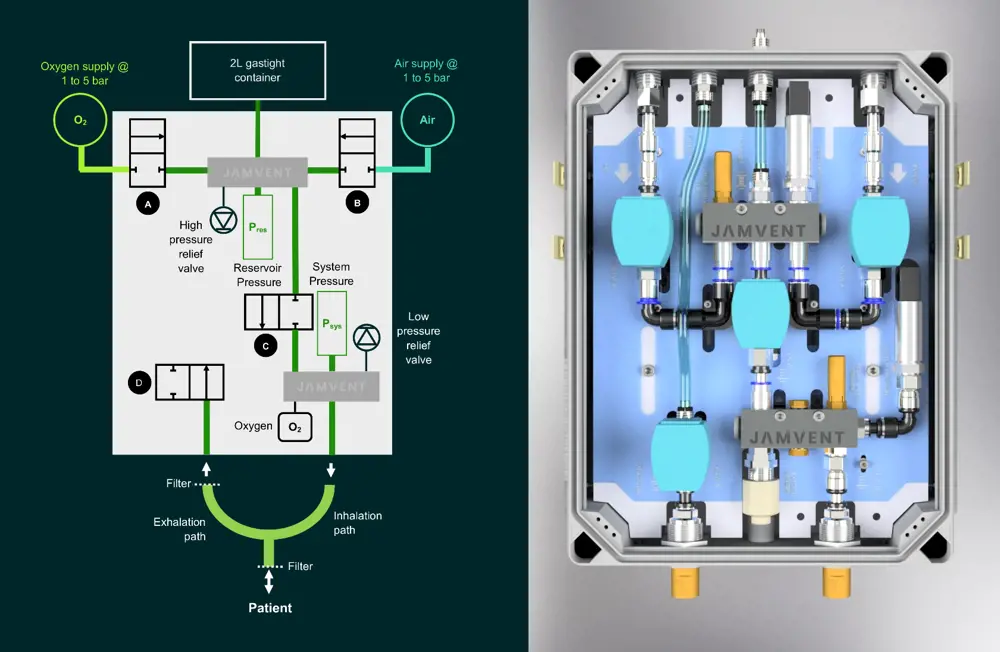
JAMVENT employs principles of fluid mechanics and uses two pressure transducers, an airtight container and a series of on–off solenoid valves to control the pressure of the air entering the patient’s lungs, avoiding the need for a balloon to pump oxygen
Other projects aiming to help underserved health systems have been developed independently. Dr Jakob Mathiszig-Lee, an Imperial College London honorary clinical research fellow and senior anaesthetic registrar at the Royal Brompton Hospital, recognised that basic functionality would not be sufficient for COVID-19 patients. He took his ideas to RAEng Research Fellow Dr Joseph Sherwood, former RAEng Research Chair in Medical Device Design Professor James Moore Jr, and Dr Michael Madekurozwa at Imperial College London’s bioengineering department. Together, they outlined clinical need and engineering requirements for a design, aiming to create a low-cost emergency ventilator model – JAMVENT – that could be assembled quickly and easily using simple components outside of the existing ventilator supply chain.
Importantly, engineers gave JAMVENT three modes: the clinically preferred pressure-regulated volume-controlled mode to deliver a desired tidal volume to the patient while controlling pressure; a mode to combine the application of suction with maintenance of pressure to keep the lungs open; and a crucial breath-sensing mode to assist patients ventilated for long periods redevelop their ability to breathe.
Compatible with hospital gas supplies and lower pressures provided by oxygen concentrators, JAMVENT delivers 100% of incoming gas to the patient and allows control of oxygen content. A key design feature of JAMVENT is that it doesn’t use proportional solenoid valves, but instead uses simple on–off solenoid valves. These are cheaper, can be provided by numerous manufacturers worldwide, and are easier to maintain. Two on–off solenoid valves control oxygen and air inlets, allowing pressurised lines to charge up the gas reservoir with a single breath of oxygen/air mixture. Reservoir charging is regulated via pressure and tidal volume requirements set by the clinician. A third valve controls when the pressurised gas mix is delivered to the patient; it also enables measurement of flow rate based on pressure drop across the valve. A final solenoid valve sits in the exhalation pathway, closing when the lung reaches a desired pressure and allowing calculation of exhaled volume.
An important element of the work was to make the design available openly
The research team developed computational models and artificial lung models to test performance, while Dr Mathiszig-Lee gave overall direction and provided answers to questions about technical requirements. The team upgraded the design to make it more modular, separating electronics and pneumatics for ease of maintenance. An important element of the work was to make the design available openly. Simple to assemble and operate, JAMVENT can be made from parts costing around £1,500 and can run from a standard PC (as well as from a medically approved touchscreen device). Parts can be sourced from various manufacturers at lower cost than proprietary components. “Each valve performs multiple functions, reducing the number of system components, improving ease of manufacture, reducing cost and enhancing supply chain flexibility,” adds Dr Sherwood.
While this design was also not taken forward by the UK government, the team is now pursuing emergency regulatory approval from the FDA, with Forbes reporting its “potential to disrupt the emerging $5 billion ventilator market”.
Engineering alternative non-invasive ventilation to help patients
As the crisis unfolded and clinicians learned more about COVID-19, evidence grew that non-invasive methods of ventilation could offer an alternative way to alleviate illness, even in those with severe infections. Among those to innovate in this area were a team from UCL that worked with clinicians and Mercedes AMG High Performance Powertrains to reverse-engineer a CPAP device, now in development as UCL-Ventura. To save time, the UCL team decided to reverse-engineer the Philips Respironics WhisperFlow CPAP device, once used widely in the NHS but now off patent. This simple mechanical device allowed rapid prototyping and mass manufacture; and using it to demonstrate like-for-like to the MHRA in terms of performance and function would help the team gain regulatory approval more quickly. It looked for a manufacturing partner and turned to the Formula 1 (F1) community, which had set up Project Pitlane to support ventilator projects. With the Australian Grand Prix cancelled and F1 in shutdown, Mercedes AMG High Performance Powertrains Managing Director Andy Cowell FREng was keen to help.
Four Mercedes engineers arrived at UCL the next day, and Mercedes ordered its own WhisperFlow from Ebay. “By 10am the next morning, this device was in our CT scanner, then we set up a test rig, and were disassembling it and looked at the tooling required,” Cowell told an online Q&A event**. To save time, one development engineer took the initiative to make adapters on his own rapid prototype machine that evening rather than wait for a new order. As well as a flow-generating device to plug into the hospital oxygen supply, other components were needed, including an oxygen analyser and breathing circuit.
We’ve learned there are a huge number of crossovers with the medical world: engineering is everywhere and this project shows our skillsets can be applied to this world in a useful way
With oxygen a limited commodity in hospitals, and the WhisperFlow relatively oxygen hungry, it was vital for the UCL-Ventura to improve oxygen utilisation. UCL’s mechanical engineers worked closely with Mercedes to redesign the entrainment port and improve flow and pressure characteristics. Mercedes set up a rig to do flow tests on filters on the PEEP valves (a spring-loaded valve that the patient exhales against), making sure the total flow was manageable with reasonable pressure drops, while its simulation team – normally busy designing inlet ports and plenums and compressors for F1 engines – worked to improve fluid flow through the jet pump part of the flow device.
Going from initial idea to prototype testing in a hospital setting took 100 hours, and approval from the MHRA took another 10 days. This came at the end of March and coincided with NHS updating its care pathway to give CPAP prominence. In one evening, an initial order of 100 devices from the Department of Health and Social Care turned into an order for 10,000. “That evening was the culmination of all of the work we’d done up to that point, an incredibly intense two weeks,” said Rebecca Shipley, Professor of Healthcare Engineering at UCL. “We shifted from development to being entirely focused on getting [the devices] out.”
Mercedes repurposed five machining centres at its Northamptonshire facility. Putting pistons and turbocharger parts to one side, manufacturing engineers reprogrammed and retooled machines with a precisely defined cycle time to meet the 1,000 a day goal. Outside suppliers manufactured the device’s three finely turned stainless steel needle valves: an on/off valve, a flow valve and an oxygen valve. This involved the challenge of ensuring drawing tolerances were robust enough for parts to go together. By 15 April, Mercedes had made 10,000 devices to meet the order. These were sent to around 60 NHS hospitals, and the UCL team has since released their designs through a controlled licence process.
One of the UCL team’s key motivations was to contribute to the international response to COVID-19. It created a compare-and-contrast between the original WhisperFlow and the UCL-Ventura device, making available drawings, CAD models, assembly and test instructions, a generic schematic for the patient circuit, and pressure versus flow rates. The blueprint has already been downloaded more than 1,800 times across 105 countries including South Africa, Bulgaria, Peru, Mexico, and Russia. There is now an ongoing effort to gather and analyse patient data.
The project also yielded benefits for Mercedes, added Cowell. “We’ve learned there are a huge number of crossovers with the medical world: engineering is everywhere and this project shows our skillsets can be applied to this world in a useful way.”
Meeting demand
😷 How the unprecedented demand for PPE and hand sanitizer was met
Many engineering businesses have repurposed equipment or pivoted production to respond to urgent needs such as Ricardo, which makes a range of complex products including engines and precision parts. At the pandemic’s outset, it settled on helping produce PPE, supporting crowd-based 3D printing consortia using rapid prototyping tools.
It then sought to scale up production, collaborating with an injection moulding partner to create a tool capable of high-volume manufacture of face shields. Applying engineering design expertise to refine the original rapid prototype design, Ricardo’s team invited local NHS doctors to give feedback. The next step was to turn two of its technical centres into temporary production lines.
Remote working (because of the need for social distancing) was one of the challenges, according to Phil Mortimer, Technical Business Manager at Ricardo Automotive and Industrial. “We completed design reviews, held supplier discussions, set up a new manufacturing line and conducted testing with a team of people working remotely.” Going from design concept to deliveries of PPE took just three weeks and the company worked with Notified Body SATRA Technology to secure fast-tracked testing of the product to the EN 166 Personal Eye Protection European Standard. Overall, Ricardo produced 10,000 protective face shields distributed to NHS hospitals, GP surgeries, care homes, and other services across the south of England and the Midlands.
As well as the unprecedented need for PPE, the crisis has generated enormous demand for another protective measure: hand sanitiser, which can help stop the virus’s spread. Hexigone Inhibitors, a member of the Royal Academy of Engineering’s Enterprise Hub, produced hospital-grade sanitiser.
Closure of construction sites meant that the firm – a chemical manufacturer that makes chromate-free corrosion inhibitors for coatings for buildings – had spare capacity in its large mixing vessels, normally used to make corrosion inhibitor. Adapting skills and resources to produce hand sanitiser was an obvious fit.
The firm set up its sanitiser manufacturing process with a team from Swansea University; the two then decided to increase output by operating independently. Via Innovate UK, Hexigone Inhibitors obtained a donation of 8,000 litres of isopropanol (the much sought-after main ingredient of hand sanitiser) from GlaxoSmithKline.
Sanitisers need to comprise 60% alcohol to be effective but Hexigone Inhibitors has made its batches to the WHO recommended formulation (75% alcohol) to ensure these eradicate coronavirus in healthcare settings. The recipe includes water (to dilute), glycerol (to moisturise), hydrogen peroxide (to kill spores) and lemongrass essential oil (as scent). The firm has now made 6,000 litres of sanitiser and is donating profits to Mental Health UK and local food banks.
Collaboration efforts in the production of PPE
While some teams raced against the clock to help patients, others focused on protecting health workers by providing personal protective equipment (PPE). Vitally needed by not only frontline healthcare professionals but also sectors including food production, retail and construction, the government took steps in March to relax regulatory requirements for a limited time to speed up the supply of essential COVID-19 related PPE on to the UK market, in line with a European Recommendation. In the case of PPE, the easement reduced the number of tests that manufacturers need to carry out on PPE products to the minimum testing considered vital for PPE being used in a COVID-19 context and a healthcare environment. The approval procedures needed for products subject to regulation, known as conformity assessment (CA), are the procedures that manufacturers follow, working with a Notified Body for the relevant regulations, in this case PPE or medical devices (or both), to show they have used appropriate standards for their products, services or systems. The evidence base the manufacturer collects enables them to claim compliance with the essential health and safety regulatory requirements, which is shown by the CE mark. In a further move to accelerate the availability of PPE, the easement allowed manufacturers that had engaged with a Notified Body to make PPE goods available for use before completing the full CA process.

CE marking of the Corran II involved making 45 prototypes, with the team using a laser cutter to form components into a complex shape and give a professional finish, with components nested on sheets to avoid wastage
Aseptium and 4c Engineering, two companies located on the same Highland enterprise campus in Inverness, shared experience in technology development and access to a suite of rapid prototyping tools. In March, they approached their local hospital to see if they could assist with PPE for healthcare workers. “The clinical lead for intensive care said that they were short of face shields, and asked if we could look at that,” says 4c’s project manager Peter MacDonald. The companies launched Project Corran, with the team considering factors including protection level, comfort, size and shape, usage frequency, and design pointers from other models, as well as regulation, sourcing non-hazardous materials, and time- and cost-effectiveness.
With an initial request to make 400 Corran I face shields, the team soon realised that – even with four 3D printers at its disposal – production wouldn’t be efficient. Instead, it opted to use components that could be sourced on the high street: clear PVC sheets, double-sided tape, foam and elastic. The aim was to make the design as easy as possible to assemble so others could replicate it elsewhere, and to use whatever resources were readily available to speed up the process. A prototype was ready within a week, which the hospital’s infection control staff approved on a Friday afternoon, and volunteers from local companies joined the production line for the weekend. By Monday, 200 shields were ready to deliver.
Importantly, the face shield was conceived and made available as an open-source design. One group in Argyll used the design to make 5,000 units, while a school-based group in Skye made 2,000 more. “It can be made with a pair of scissors but people can also automate elements of manufacture,” MacDonald explains. The Scottish consortium had itself produced 7,800 shields by the end of April.
Ongoing and urgent need for PPE meant the project soon entered a new phase. Prototypes were engineered not just to meet regulatory requirements but also to improve wearer comfort. Little changes had a big impact: Corran mark II incorporates tabs to hook up a mask to reduce pressure on the wearer’s ears. The bend of the visor holder was also engineered to provide maximum comfort and ease of manufacturing, and modifications allowed components to be cleaned and reused. After testing by Notified Body SATRA Technology, Corran mark II is now a CE marked piece of PPE.
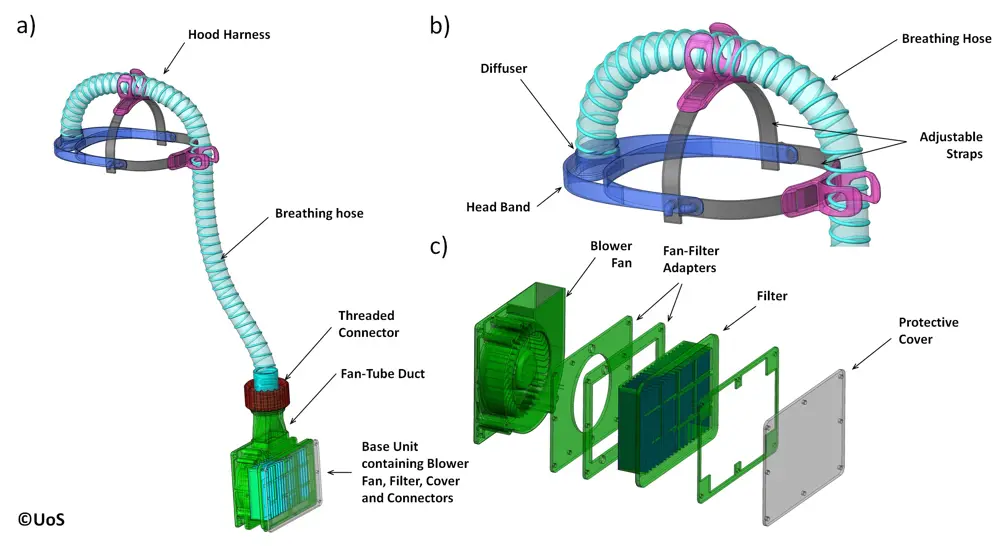
The second prototype for the PeRSo was presented to University Hospital Southampton in late March and pilot testing led to further modifications © University of Southampton
Another example of rapid innovation comes from a team that created a new form of powered air-purifying respirator (PAPR). A PAPR offers better protection than a respirator by using a fan to draw in air through a filter and channel it via an enclosed hood or mask. However, expense, noise, heaviness, and discomfort makes some models impractical for lengthy wear. The concept for a new version – now being manufactured as PeRSo – came from Professor Paul Elkington, Professor of Respiratory Medicine at the University of Southampton. Seeing the pandemic unfold, he realised clinicians involved in high-risk aerosol-generating procedures such as intubation would need a more comfortable respirator.
He took his ideas to colleagues including Professor Hywel Morgan, who led formation of an engineering team that included Dr Alex Dickinson, a RAEng Research Fellow and Associate Professor in the mechanical engineering department. “Within the week, we had a working prototype we took to University Hospital Southampton,” says Dr Dickinson. “They gave us helpful feedback on our first prototype, and by the following Thursday we returned to them with a mark II.”
Prototyping involved verifying the basic function in terms of filter efficiency by assessing bacterial growth on an agar plate inside the respirator, and looking at its ability to filter out droplets of a bitter-tasting chemical commonly used in fit-testing mask respirators. The team did extensive pilot user testing.
The result is the lightweight PeRSo, consisting of a fabric hood, plastic visor, small portable unit delivering clean air through a high efficiency particulate air (HEPA) filter, and battery-powered fan mounted on a belt pack. PeRSo also shows the wearer’s face, increasing comfort for both wearer and patient.
he realised clinicians involved in high-risk aerosol-generating procedures such as intubation would need a more comfortable respirator
Dr Dickinson highlights decisions made in relation to projected use of PeRSo, considering materials, distribution of weight to prevent neck muscle fatigue, restriction in movement, and ease of cleaning and decontamination. Local firm INDO Lighting took PeRSo into further development, testing and manufacture, and the first 4,000 have been deployed at University Hospital Southampton with 6,000 more ordered for use across Hampshire. There is an active application in place for regulatory approval and the PeRSo is currently undergoing testing at BSI.
As with Project Corran, this project’s key elements included the use of commonly available materials and making the design available to others. “We published an Open Specification as early as we could, and followed with full open-source design files once the prototype was more refined,” reflects Dr Dickinson, who is now part of a team developing PeRSo for lower- and middle-income countries. “It was also important to collaborate, bringing in support at the right stage, such as partners for testing and large-scale manufacturing.”
In extraordinary times, the engineering profession has contributed to the COVID-19 response through many more innovative projects and multidisciplinary efforts. The efforts of these engineers and organisations are just a fraction of the ways in which the community has mobilised to rapidly add expertise to the ongoing battle against the virus, as well as learning lessons from innovating and scaling at pace.
***
Information and quotes about the Ventilator Challenge UK consortium were taken from an online Q&A event, organised by the Royal Academy of Engineering, with Dick Elsy CBE, Chief Executive of the High Value Manufacturing Catapult, and Dr Graham Hoare OBE FREng, Executive Director (Business Transformation) & Chairman, Ford of Britain, on 29 May 2020.
nformation and quotes about the UCL-Mercedes Ventura device were taken from an online Q&A event, organised by the Royal Academy of Engineering, with Professor Rebecca Shipley, Professor of Healthcare Engineering, Director of the Institute of Healthcare Engineering and Vice Dean (Healthcare), University College London and Andy Cowell FREng, Managing Director, Mercedes AMG High Performance Powertrains, on 19 May 2020.
This article has been adapted from "Responding to a global pandemic", which originally appeared in the print edition of Ingenia 83 (June 2020).
Contributors
Rachel Jones
Author
Keep up-to-date with Ingenia for free
SubscribeRelated content
Mechanical

When will cars drive themselves?
There are many claims made about the progress of autonomous vehicles and their imminent arrival on UK roads. What progress has been made and how have measures that have already been implemented increased automation?

R&D investment makes good business sense
In just five years, Dr Ralf Speth FREng has presided over a revolution in design and manufacturing that has helped create a new family of engines and has overhauled Jaguar Land Rover (JLR) production facilities.

Bikes help improve skills and attitude
The Archway Project is an independently-funded scheme that is expanding its engineering-based programmes by providing BTEC certificates and diplomas. John Milton, the director of the project, explains what the charity does to help reduce anti-social behaviour and improve employment prospects.

High speed evolution
In December 2010, Eurostar International Ltd awarded a contract for 10 new high speed trains to Siemens. The company has used a system developed over decades to maximise the performance and passenger-carrying ability of its 320km/h trains.
Other content from Ingenia
Quick read

- Environment & sustainability
- Opinion
A young engineer’s perspective on the good, the bad and the ugly of COP27

- Environment & sustainability
- Issue 95
How do we pay for net zero technologies?
Quick read

- Transport
- Mechanical
- How I got here
Electrifying trains and STEMAZING outreach

- Civil & structural
- Environment & sustainability
- Issue 95