
Fuel for a net zero future
Did you know?
⛽ Synthetic petroleum and renewable energy
- Petrochemicals are vital to many parts of everyday life, including pharmaceuticals, clothing and energy
- A synthetic petroleum can be created using chemical engineering to create an alternative to fossil-derived fuels
- Using renewable energy, this chemical process could create a fully circular system
This is the dawn of a new Industrial Revolution. For 300 years, since the rise of coal mining and drilling for fluid hydrocarbons, we have relied on fossil energy and increasingly on oil for fuels such as petrol, diesel and jet fuel, and for much of our industrial material, such as plastics, textiles, fertilisers and pharmaceuticals.
Despite the drive for a net-zero planet, human nature will make it hard for society to reduce its energy consumption. More and more people expect to be warmer, cooler and healthier and to travel further, faster and more often. Meanwhile the world’s population continues to increase and those in low- and middle-income countries should be able to enjoy the same benefits as those in higher-income countries.
One way to meet our demand for energy that does not exacerbate climate change is to change our linear system of consumption to a circular one, with an indefinitely sustainable supply that does not add CO2 to the atmosphere.
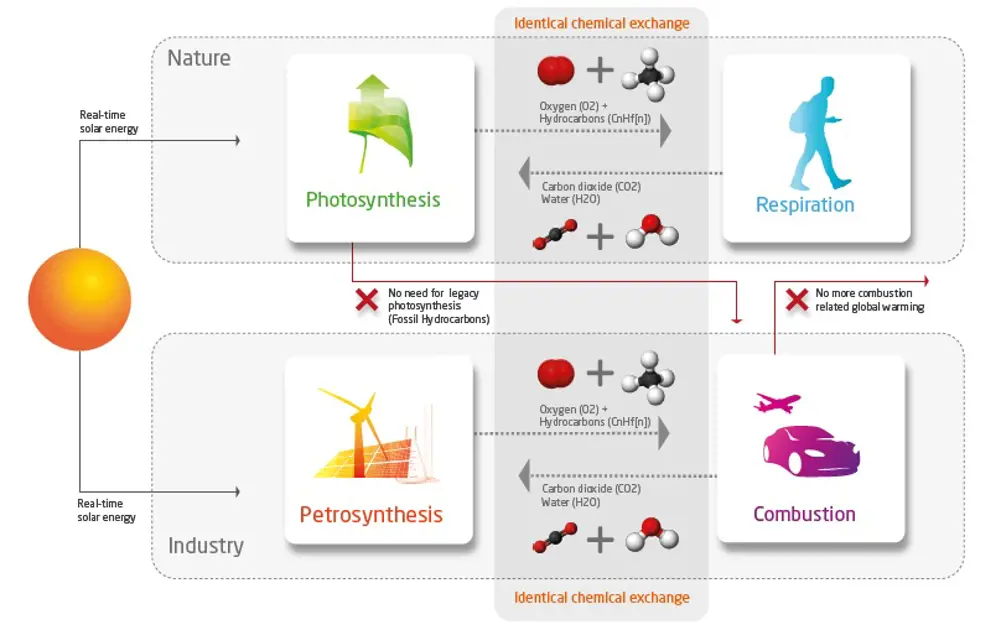
Petrosynthesis can complete the industrial carbon cycle - in this example using solar energy, but other forms of renewable energy can also be used © Zero Petroleum
Renewable energy sources, electrification and the development of hydrogen fuels are already addressing this need. But we could further advance this progress towards net zero with synthetic petroleum, a form of petroleum that does not involve fossil hydrocarbons. Petrosynthesis entails a combination of chemistry and chemical engineering in processes that reverse what happens when we burn petroleum, when hydrocarbons combine with oxygen to create water and CO2. The use of renewable energy to power petrosynthesis then creates a fully circular and sustainable system, which is a scalable substitute to fossil-derived petroleum fuels and materials.
Petrosynthesis is the artificial creation, or synthesis, of organic chemicals, such as hydrocarbons and oxygen from inorganic precursors, principally water and CO2. The idea is to use non-biological energy, such as hydro, wind, solar, tidal, nuclear, or geothermal energy, to power the process. It is the industrial equivalent of photosynthesis, using neither energy nor material produced by either concurrent photosynthesis (plants) or legacy photosynthesis (fossil fuels).
The balance of synthesis in nature
Synthesis is a common process in nature. All life on Earth employs carbon, oxygen and hydrogen as primary chemical elements. Plants create hydrocarbons from solar energy by photosynthesis and animals burn off these hydrocarbons through respiration, with the atmosphere working as a huge storage tank for the ingredients.
This circular process is the so-called ‘carbon cycle’. It worked perfectly for hundreds of millions of years, with only a very marginal imbalance, which provided the hydrocarbon deposits known as fossil fuels. When society could access these fuels in combination with energy-consuming technologies, it led to exponential development.
Fossil energy and materials are finite resources, consumption causes imbalance and waste – the current model is unsustainable.
The Industrial Revolution changed everything. Fossil fuels drove scientific and engineering developments with immeasurable benefits, including health, mobility, food cultivation, and human comforts. Today’s industrialised society now has an almost total dependence on petroleum for energy and materials.
Industrial combustion has the same macro-chemistry as respiration, but as fossil fuels are the product of legacy photosynthesis, rather than being part of an ongoing process, it has turned life on Earth from a circular system into a linear system. Fossil energy and materials are finite resources, consumption causes imbalance and waste – the current model is unsustainable.
The performance gap between renewable energy and fossil fuels
Fossil fuels have enabled development of ‘sustainable’ technologies, for example from sun, wind, hydro, nuclear fission, and eventually nuclear fusion. These create the carbon-free electricity that will form future power systems. However, for some industries, renewable electricity alone will simply not work.
Battery technology for storing and moving electrical energy has advanced and hydrogen has emerged as a solution where electricity is unsuitable. However, the energy density in batteries is typically 50 times lower than current liquid fossil fuels, accounting for the differing efficiencies of electrical and combustion motors. The energy density of hydrogen contained in a tank is better than a battery but is still typically four times below that in fossil fuels.
These factors leave a critical performance gap for a huge range of common and vital assets to the industrial system. Aeroplanes, military vehicles, helicopters, fast boats, and high-performance cars all need a fuel with a relatively high energy density. Perhaps surprisingly, agricultural machinery also lies within this list, most notably the tractor and the combine harvester, arguably the most important machines to have enabled humankind’s release from a fully agrarian society.

The plane and pilot that hold the Guinness World Records® title of the ‘first aircraft powered by synthetic fuel’ © Zero Petroleum
We will need high-density energy stores in the future, yet the current spectrum of sustainable energy resources does not account for this. Fundamental chemical and electro-chemical constraints mean that technical development is unlikely to close the mass and volume deficiencies of electrical and hydrogen energy stores.
While natural materials are making a comeback, petrochemicals remain vital to many parts of everyday life, from pharmaceuticals to clothing. It will be extremely challenging to overcome our reliance on them, economically and functionally. As a result, petrochemicals remain important: we need fossil-free alternative
With the support of the Royal Air Force, in just five months Zero Petroleum built from scratch and operated a plant to manufacture synthetic aviation gasoline, culminating in a manned flight in November 2021. This flight earned the Guinness World Records® title of ‘first aircraft powered by synthetic fuel’. The fuel delivered identical power without any modification whatsoever to engine or aircraft. The company opened the first commercial-scale synthetic fuel plant in the UK in June 2023, where a synthetically fuelled model jet cut the opening ceremony ribbon.
Petrosynthesis: an industrial version of the carbon cycle
Petroleum is simply a set of chemicals, hydrocarbons, that we use to produce fuels such as gasoline, diesel and jet fuel and to create the precursors for plastics and many other modern products. Biological substitutes have been developed over decades. These include bioethanol and biodiesel, but they will continue to remain a niche solution at best. A typical field crop is 22 times less efficient than a solar panel in terms of the ‘energy productivity’ per hectare. As a result, many biofuels are marginal, if not negative, in terms of net energy after cultivation and processing. Even second-generation biofuels that use biological waste are energy and water intensive. Fuels that use petrochemical waste do help to reduce contamination, but they are still fossil derived, so simply defer emissions.
Direct air capture (DAC) can remove CO2 from the air so that it can be stored in a process called carbon capture and storage. This stores CO2 underground, for example using mineralisation in basalt rock to sequester carbon into geologic formations. It is therefore still a linear and unsustainable solution with no role beyond a transitionary offset for continued carbon emissions from fossil fuels or to mitigate past carbon emissions by fossil fuels. Reuse or recycling of atmospheric carbon as a raw material for new products is a much more exciting application of DAC.
It is the industrial equivalent of biological photosynthesis. Combustion of its products is the equivalent of respiration in animals; this produces the raw CO2 that feeds the circular process.
Companies like Blue Planet, Solidia, CarbonCure, and CO2 Concrete are converting captured CO2to create concrete. The company C2CNT creates carbon nanotubes that are stronger than steel and highly conductive. If scalable, these technologies have the potential to transform buildings and electrical circuitry.
Similarly, petrosynthesis uses CO2 from DAC to produce synthetic petroleum for fuels and petrochemicals. It is the industrial equivalent of biological photosynthesis. Combustion of its products is the equivalent of respiration in animals; this produces the raw CO2 that feeds the circular process. Petrosynthesis creates an industrial version of the carbon cycle in biology.
Fossil-free processes in practice
Termed ‘a forest in a factory’, petrosynthesis can create fuels, plastics, rubber, textiles, and any other hydrocarbon. It is not ‘zero carbon’ but it is net zero and fossil free. The process is being applied at an increasing rate. Climeworks, a leader in DAC engineering, recently opened the world’s largest facility in Iceland. In this plant a filter captures CO2 from the air, combines it with water and pumps it 600 metres into the ground where it reacts with rocks. The US company Global Thermostat is doing things differently; rather than burying CO2, it provides it to customers for industrial processes, from fizzy drinks to plastics manufacture.
Termed ‘a forest in a factory’, petrosynthesis can create fuels, plastics, rubber, textiles, and any other hydrocarbon. It is not ‘zero carbon’ but it is net zero and fossil free.
The UK company Zero Petroleum has its focus on developing fossil-free petrol, diesel and jet fuel. Founded by Paddy Lowe FREng and Professor Nilay Shah OBE FREng in January 2020, it is the only company operating commercially in the UK to design and manufacture synthetic hydrocarbon fuels. In 2020, it obtained support from Innovate UK for laboratory R&D work to develop synthesis technology. Also being developed by Norsk e-fuel and Porsche, these fuels can be made at scale and used in existing engines as a direct drop-in replacement for fossil fuels. They have the same carbon emissions when burned, but through DAC the carbon is recycled into the production of more fuel, creating an indefinitely sustainable supply. Zero Petroleum has developed a process that uses renewable energy to power an electrolyser, which turns water into hydrogen, along with DAC to take CO2 from the air. A reverse water–gas shift reactor converts the CO2 into carbon monoxide. This combines with hydrogen to create a ‘syngas’ that feeds a Fischer–Tropsch (FT) reactor to create the final hydrocarbon products (‘Biofuels journey to the mainstream’, Ingenia Issue 78).

Virgin polyester fabric made from recycled carbon dioxide is being used by sportswear companies to make clothing, addressing the issue of sustainable production © Lululemon
To produce 1 kilogram of synthetic hydrocarbon, the process uses 1.46 kilograms of water and 3.07 kilograms of CO2. The electrolysis produces 3.53 kilograms of oxygen. When the synthetic fuel burns it produces the same quantities in reverse.
Some companies are developing ways to harness the sun’s power directly as heat without using renewable electricity, such as concentrated solar power. For example, Heliogen and Synhelion use aligned mirrors to reflect sunlight onto a target to create extremely high temperatures within a chemical reactor. The reactor processes atmospheric water and CO2 first into syngas (hydrogen and carbon monoxide) and subsequently into hydrocarbon fuels by a similar FT process to that used by Zero Petroleum. They call these ‘solar fuels’, which are an alternative example of petrosynthesis.
Petrosynthesis will also change how companies make the things we wear and the products we use in our everyday lives. The company On is making foam cushioning for footwear for running shoes and yarn using recycled CO2. The sportswear company Lululemon makes clothes with fabric that uses carbon from waste materials rather than fossil carbon. Both companies are using carbon recycling technology developed by LanzaTech. This is just the start of circularity, by petrosynthesis, in the clothing sector, which is under pressure to develop sustainable product
This is just the start of circularity, by petrosynthesis, in the clothing sector, which is under pressure to develop sustainable product.
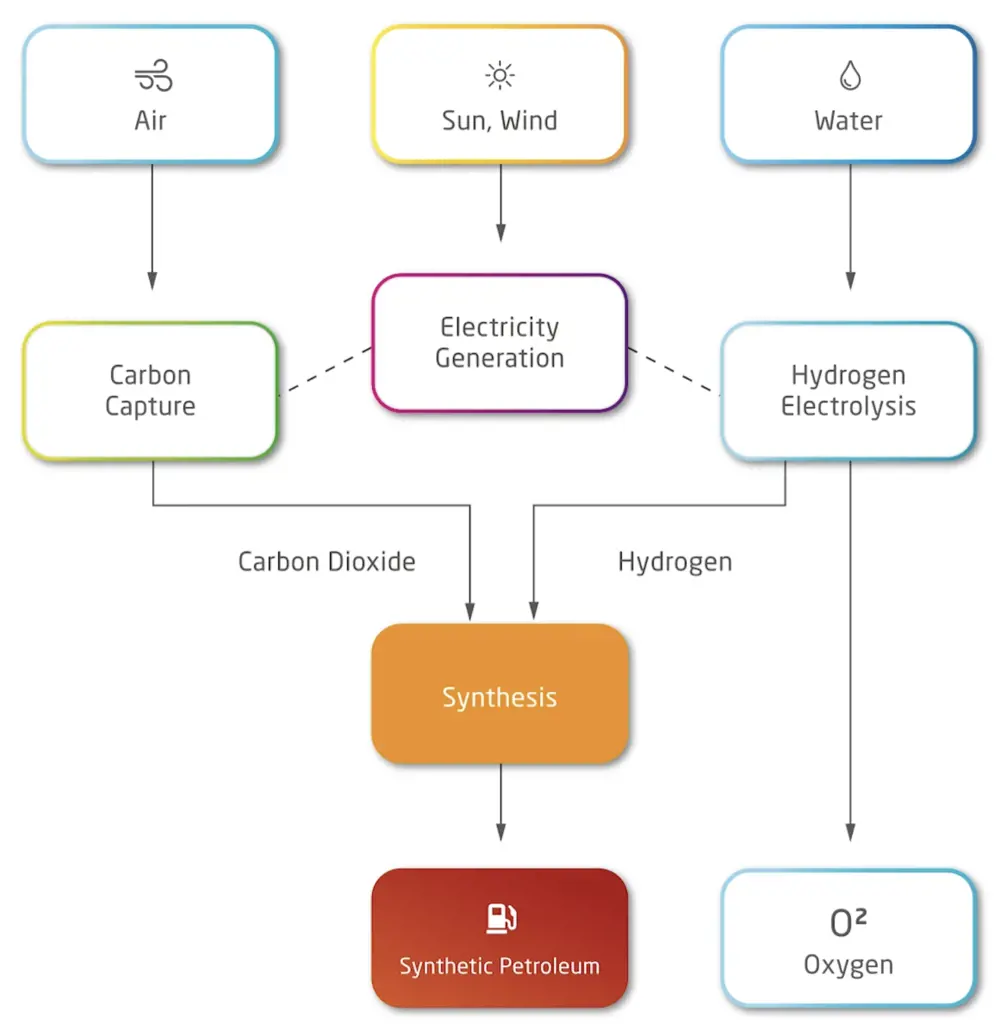
Manufacture of synthetic petroleum via the Fischer-Tropsch process © Zero Petroleum
How to make synthetic petroleum
Synthetic petroleum can be made by several chemical pathways. The classic Fischer–Tropsch process, developed in Germany nearly 100 years ago, uses catalysts (typically iron or cobalt) at temperature (300 to 400°C) and pressure (20 to 40 bar) to promote the conversion of carbon monoxide and hydrogen (originally produced by the gasification of coal) into hydrocarbons and water. This is an exothermic reaction. The challenge is to produce the desired hydrocarbons and with the highest possible yield. More modern catalysts such as zeolites are providing these improvements. Within the context of renewable energy, the focus is to obtain best process efficiency as all the hydrogen (green hydrogen) must be expensively generated from renewable electricity by the electrolysis of water. Within petrosynthesis, the source carbon is atmospheric CO2, which may be converted in a front-end process to carbon monoxide also by electrolysis, or by another catalytic reaction called reverse water gas shift (RWGS), which strips the oxygen using more green hydrogen and producing more water.
How petrosynthesis will fit into our future energy system
Petrosynthesis is not an interim or transitional technology. It will be an essential component of our future fully sustainable energy system. For example a 2020 Ricardo report, Renewable electricity requirements to decarbonise transport in Europe with electric vehicles, hydrogen and electrofuels, shows that by 2050 between 84% and 100% of European commercial aircraft will be powered by eKerosene, a type of synthetic fuel. Combining electricity, hydrogen and synthetic petroleum can create a circular energy economy. This is net zero, with each energy format offering its own merits for different applications, each adopted according to performance imperative and/or economics.
Direct electricity is the cleanest and most efficient energy format but it is the hardest to store and the most difficult to distribute. Hydrogen has a medium rating on all factors, clean, relatively efficient at storing energy, and moderately easy to distribute. Synthetic petroleum is the densest energy format, easiest to store and easiest to distribute but the least clean and efficient. These three energy formats can work symbiotically, with excess electricity generated from renewable energy used to create hydrogen and synthetic petroleum, some of which is used in gas-fired power stations during times of darkness without wind.
Renewable electricity requirements to decarbonise transport in Europe with electric vehicles, hydrogen and electrofuels, shows that by 2050 between 84% and 100% of European commercial aircraft will be powered by eKerosene, a type of synthetic fuel.
As an efficient industrial process, petrosynthesis can be scaled up to meet global demand for petroleum while preserving fields and forests for agriculture and nature. Because it is synthetic, petrosynthesis can be refined to create better products, eliminating the impurities in raw fossil fuels to reduce the effect of emissions of particulates, carbon monoxide and nitric oxide.
The technology has been proven to work. The engineering challenge is to keep pushing the boundaries of overall energy efficiency and the quality and yields of products. Process optimisation will need a wide range of engineering specialisms, from material science and catalysis, through to advanced simulation and modelling of thermodynamics and fluid dynamics.
Petrosynthesis, the 21st century’s engineering equivalent of the natural carbon cycle, could be a fundamental pillar of future industrial systems. In 1,000 years, people will look back at this transition and understand that fossil fuels were not bad; they were just a vital windfall and stepping stone on the road to human development of a circular civilisation.
***
This article has been adapted from "Fuel for a net zero", which originally appeared in the print edition of Ingenia 89 (December 2021).
Contributors
Paddy Lowe FREng started his engineering career in Formula One in 1987 after graduating from the University of Cambridge. He is widely recognised for his achievements as an innovator and technical director, including leading the Mercedes team to the most successful season ever recorded: 19 wins from 21 races in 2016. He is the founder and CEO of the synthetic fuel company Zero.
Professor Nilay Shah, OBE FREng is one of the most significant and influential chemical engineers within global academia. He has a PhD in chemical engineering from Imperial College London and is Professor of Chemical Engineering at the university. Professor Shah has received numerous awards, and has a track record of strong industrial engagement and consequent commercial success for research activities under his leadership.
Keep up-to-date with Ingenia for free
SubscribeOther content from Ingenia
Quick read

- Environment & sustainability
- Opinion
A young engineer’s perspective on the good, the bad and the ugly of COP27

- Environment & sustainability
- Issue 95
How do we pay for net zero technologies?
Quick read

- Transport
- Mechanical
- How I got here
Electrifying trains and STEMAZING outreach

- Civil & structural
- Environment & sustainability
- Issue 95