
Delivering fast-track COVID-19 vaccines
The COVID-19 pandemic has already caused an estimated 1.3 million deaths and more than 200 groups around the world are working to develop vaccines to stop the virus’s spread. With the molecular blueprint of the virus published in January, phases of development that normally take years have been overlapped in the push to deliver a vaccine as soon as possible. Leading candidates include a project backed by the University of Oxford and AstraZeneca, which takes a ‘viral vector’ approach that uses a virus to carry the vaccine into the body. Two vaccines currently leading the race, developed by Pfizer/BioNTech and US biotech firm Moderna, are reported respectively to show 90% and 95% protection*. Both use an mRNA approach, with ‘messenger’ ribonucleic acid (RNA) – sets of instructions for cells to make proteins – preparing the immune system. Imperial College London is taking an alternative approach, using self-amplifying RNA to produce copies of itself that can instruct cells to make the coronavirus protein.
Addressing the task of establishing vaccine manufacture and supply
Establishing vaccine manufacture and supply is a complex task, with governments, industry and other stakeholders working together to share risks and costs. In the UK, pandemic-related shortages and delays are presenting new challenges alongside a backdrop of a gradual decline in UK vaccines expertise and manufacturing capacity. Developing vaccines has low profit margins and, as vaccines are given to healthy people, the regulation process is especially stringent.

A CGI rendering of the eventual Vaccines Manufacturing and Innovation Centre (VMIC) site in Harwell, Oxfordshire. An important aspect of VMIC’s work will be training new generations of engineers and technicians. Undergraduate and postgraduate industrial placements, continuing professional development and support for those mid-career will create opportunities not just in the UK but also in the international vaccines community © VMIC
The Vaccines Manufacturing and Innovation Centre (VMIC) has set up a temporary rapid deployment site to provide extra capacity to develop and manufacture vaccines. VMIC is a not-for-profit vaccine manufacturing organisation established by the University of Oxford, Imperial College London and the London School of Hygiene and Tropical Medicine, and largely funded by UK government. Its main site at the Harwell Science and Innovation Campus in Oxfordshire will open in mid-2021. It will develop and manufacture vaccines that need differing approaches, including production to take candidate vaccines up to large-scale manufacture.
VMIC has established a temporary site in Oxford, in collaboration with gene therapy firm Oxford Biomedica, to become one of the producers of the Oxford/AstraZeneca vaccine. A ‘virtual VMIC’ comprises two manufacturing suites approved by the necessary regulatory bodies.
A risk-based approach: clinical trials and vaccine approval
Investing in manufacturing processes during clinical trials means that vaccine developers are ready to ship sufficient quantities as soon as a vaccine receives regulatory approval. Clinical trials begin with a small study of healthy people to evaluate safety and immune responses at varying doses (Phase I). The trials then progress through larger safety and efficacy studies (Phase II). The vaccine is studied in thousands of people (Phase III). A regulatory review of the results by the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK follows. However, this step can take place after manufacturing begins but there is no guarantee of efficacy or approval. “I would expect the regulators to know about trial progress and outcomes as they occur so they can carry out a rolling review,” says Dr Donal Cronin FREng, an engineering and safety consultant and formerly head of AstraZeneca’s global engineering and technology organisation. “When final data from the trial are available, the regulators can then complete approval processes quickly.”
If approval is delayed, a vaccine made in bulk may go to waste; conversely a vaccine could be approved before there is time to make enough product. Ideally, a candidate vaccine is manufactured in bulk during trials, wins approval, and is then distributed widely. Groups putting vaccine candidates into production will take a risk-based approach. The bigger the risk level – or, in this case, global need – the sooner production begins. In some cases, the need is so high that large-scale manufacture begins in parallel with Phase I trials. Depending on manufacturing approach, technology and scale, the time taken to produce batches can vary considerably, as can the quantities of vaccine produced. Supply chains will be primed as soon as approval comes, with tens of millions of doses – made ‘at risk’ prior to approval – ready to be released.
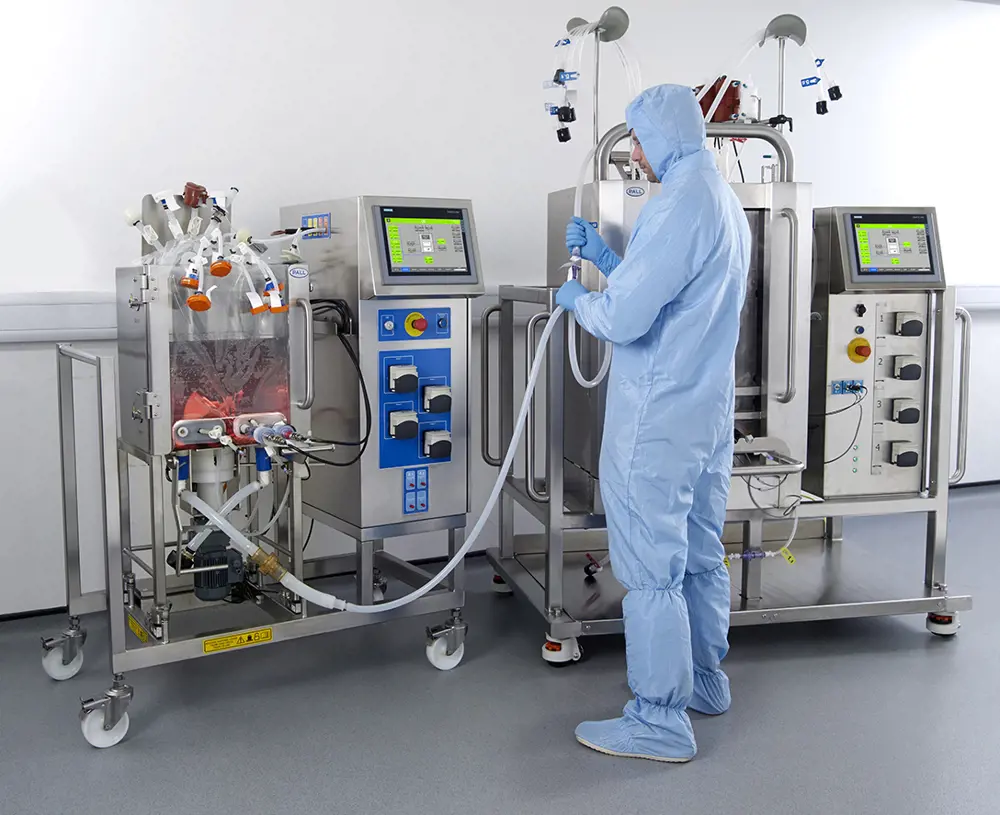
Bioreactors are an example of the specialist manufacturing equipment the VMIC has purchased for its virtual VMIC facility, such as these Allegro™ STR bioreactors 50 L and 200 L © Pall Corporation
Different platforms to allow rapid manufacture
The first vaccines to be approved may be the most welcome, but they may not remain the most effective. “The COVID-19 virus is slow to mutate but any group would be remiss if it wasn’t looking at this issue of mutation,” says Dr Cronin. AstraZeneca’s viral-vector platform may be the focus for the UK’s virtual VMIC but no-one knows which winners will emerge in the long term. To cater for different vaccine platforms, VMIC’s main Harwell site is “technology agnostic” and can use different technologies to solve different problems.
VMIC anticipates being able to work at laboratory and intermediate scale on any major vaccine platform. Martin Smith, Head of Manufacturing Sciences and Technology at VMIC, explains that the facility is preparing to work on as many platforms as possible. “Engineers are trained in lateral thinking, how to best appropriate one technology for another purpose… The challenge lies in choosing and installing the most appropriate and flexible equipment that will allow us to do rapid process development, and robust and flexible manufacturing across the scale.”
VMIC is receiving support in kind from pharmaceutical companies including Janssen Pharmaceuticals, MSD Ltd, and Cytiva Ltd, and will work closely with industry, to leverage understanding of currently available production technologies. Transfer of processes is another challenge, as companies may possess equipment with differing characteristics. The key to responding rapidly to outbreaks of disease, such as the current pandemic, lies in understanding these differences and assessing risks when scaling processes ‘up’ (in volume) or ‘out’ (into multiple bioreactor units, which carry out a biological reaction or process on an industrial scale). Some risks can be addressed through small-scale experimentation such as mixing studies – analysis of flow patterns resulting from mixing within a bioreactor to ensure sufficient mass-transfer is available without harming delicate cells. These may be needed for different types of bioreactors.
Cutting complexity will help to reduce risk, says Smith – and this is made easier by having all operations on a single site. “We not only have process development for the design of the manufacturing process, but we can transfer that technology and remove bottlenecks. All functions are under one roof with a mapped out end-to-end process.” VMIC can add value, he says, in its “complete understanding of the value chain, from raw material to the filled vial”. The Harwell site will make vaccines in bulk and fill vials in one facility, using the same personnel, quality management system and warehousing, removing risk by reducing interfaces and interactions that take time and add complexity.
How do vaccines work?
💪Training the immune system to fight off viruses and bacteria
Vaccines work by training our bodies to recognise and respond to proteins that are produced by disease-causing (pathogenic) organisms, such as viruses or bacteria. When weak germs are injected into the body, the portion of the pathogen known as the antigen trains the immune system to respond to infection by creating antibodies. Of 236 vaccine candidates being developed for COVID-19, some take traditional approaches to vaccine development, using inactivated virus or attenuated pathogens. Another popular approach uses part of a virus rather than the whole virus. Some projects employ a virus-like non-infectious particle that cannot replicate and contains no viral genetic material, offering a safer alternative to attenuated virus.
Typically, a vaccine contains an antigen or adjuvant that provides immunity against a disease. Antigens are obtained from the structure of disease-causing organisms: the immune system recognises these as ‘foreign’, triggering a protective immune response to the vaccine. Adjuvants are added to vaccines to stimulate the production of antibodies against the vaccine to make it more effective. A vaccine will also contain: preservatives and stabilisers to keep the vaccine safe and stable; production materials such as cell-culture material; antibiotics like neomycin to help stop outside germs and bacteria growing in the vaccine; and inactivating material such as formaldehyde, which can weaken or kill viruses, bacteria or toxins in the vaccine.
In manufacture, creation of a validated and reproducible production process – a basic requirement for any regulated medicine – is the overriding challenge. Essentially, this means that all ingredients must be characterised, registered, available, and procured at a reproducible and defined standard.
Production of fast-track vaccines
Production processes are an important factor in the development and manufacture of any vaccine. They are also subject to regulatory control. Any alteration to the manufacturing process has implications for product quality. There are numerous variables in any production process, including time, temperature and agitation conditions. To manage these, the production process must be recorded and understood thoroughly. Developers must implement production controls, and all equipment and services must be well defined and validated.
Registered production processes must then be replicated exactly in full-scale production. “When a manufacturer establishes production at larger scales to meet demand, it cannot modify a purification technique or substitute a raw ingredient without retesting or re-approval,” says Dr Cronin. “From a medical point of view, any deviation could introduce the risk of producing a slightly different product. Manufacturers must be true to the original process or face going through the approval process again. This ties your hands in some respects but calls for greater ingenuity to meet increasing demand.”
To make fast-track vaccines, manufacturers will overlap normally sequential activities. Production begins with generating an antigen to create an immune response. Viruses can be grown in primary cells such as chicken eggs or on continuous lines of host cells recognised for their ability to grow and develop various biomedical species. Recombinant protein (where genetic material comes from multiple sources in a lab), deriving from viruses or bacteria, can be generated in yeast, bacteria, or cell cultures.
To make fast-track vaccines, manufacturers will overlap normally sequential activities
A production-scale single-use bioreactor is essentially a multi-layer, disposable plastic bag, produced in sizes up to 5,000 litres. This sits in a frame that rocks to produce a gentle wave, with the surface resting on a heated plate to control temperature. In viral production processes, the ability to create a fast turnover of product batches means single-use products can maximise output and reduce investment. The removal of certain utilities means a smaller area for production is required and a simpler process.
For COVID-19 vaccines, single-use technologies will create flexible, agile, accessible manufacturing. Dr Cronin predicts that most companies will use this approach for cell growth and harvesting. With preparation for production of vaccines on a mass scale, there could be a shortage of disposable technology. “Everyone will be looking for single-use tubing, bioreactor bags, and all the paraphernalia to regulate and control temperature and other manufacturing parameters.”
Once generated, the antigen is isolated from the cells used to generate it, and then purified. The desirable component is usually extracted through centrifuging, sometimes followed by other separation techniques. Chromatography may then be used as a separation process to remove impurities. The purified antigen is then combined with adjuvants, stabilisers and preservatives (and perhaps emulsified) to form the final preparation.
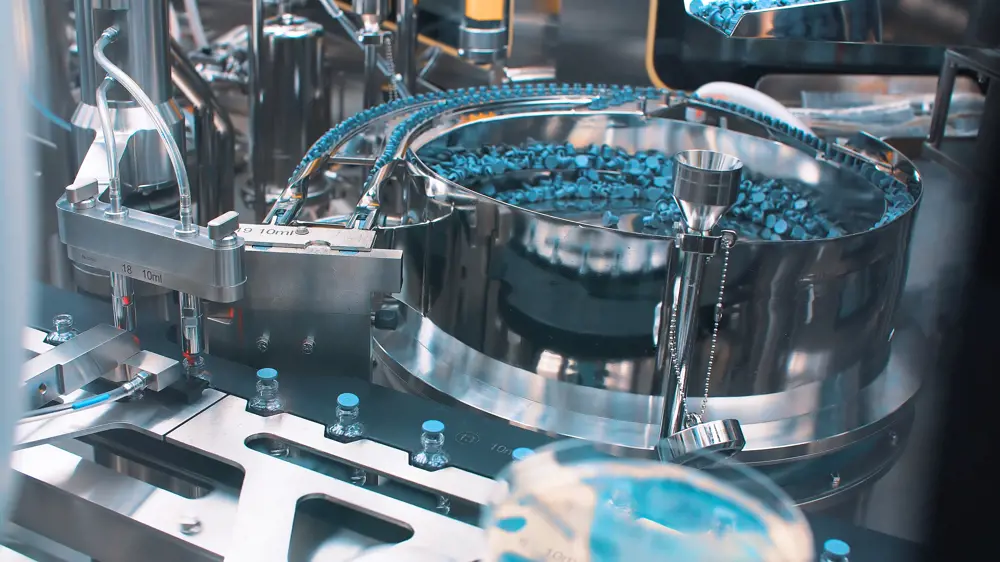
Vaccine manufacture will consist of automatic packaging machines such as this for packaging ampoules with vaccine, injection and medical drugs © Shutterstock
Typically filled in single-dose or multi-dose vials, a vaccine preparation is freeze-dried and sealed, removing water to leave a powder that can be dissolved into liquid again. Freeze-driers are complex items with a finite number of suppliers, so obtaining these could represent another challenge, says Dr Cronin. “You might be looking at a lead time of 16 months for them.” In some instances, bulk vaccine is supplied in liquid form to dispensaries, with small volumes taken off for daily clinics. This needs a less complex manufacturing process, but the vaccine may have a shorter shelf life and may involve a more demanding supply chain.
Supply chain pressures during the pandemic
Pressures on supply chains for equipment and components have increased during the pandemic. Strategic decisions on supply chain, such as locations and personnel for manufacturing, formulation, filling, and final packing, and for transportation and distribution, need to be made well in advance before a vaccine candidate reaches formulation and packaging. One option is to locate manufacturing formulation and packing in one place and set up global supply chains to deliver vaccines. A single centre of expertise may help control quality and compliance but a centralised model carries risks, says Dr Cronin. “It’s likely to be highly complex, lack agility, and create variable delivery times to get the vaccine to end points.” For example, if an entire global supply comes from one source, delivery to adjacent countries is likely to be much faster than to countries on the opposite side of the world (taking into account travel time, multiple customs clearances, and so on), which will affect shelf life and stability.
VMIC’s permanent facility at Harwell will establish processes to manufacture up to 70 million doses of a pandemic vaccine within four to six months, with specified and procured filling and packaging lines. “Our filling lines are technology agnostic,” says Chris Lucas, Chief Operating Officer at VMIC. “We will formulate a drug substance to the right presentation and send it to the filling suite to be put into vials and passed to a packaging line. From there it can be sent on for distribution.”
Dr Cronin believes producers will create in-region or in-country distribution hubs, with vaccine products sent from factories to logistics partners offering temperature control warehousing and guaranteed security. From there, once local drug administration authorities give final approval, the vaccines will flow to governments and private entities, such as charities and private healthcare.
Quality control and security in pharma
Distribution is the next step. Quality control and assurance tend to be well-established and well-resourced processes in major pharma companies. For every five people involved in production, one will be in quality management. “Together with analytical specialists, this tends to be the most intensively staffed part of the production chain,” says Dr Cronin. Quality assurance culminates in batch release, with doses dispatched into the distribution chain. Warehouses, lorries and transfer procedures then need to retain products within a carefully controlled temperature range.
Irrespective of how the vaccine is supplied, development consortia must ensure supply chain integrity, not just security of supply but security of the chain itself. COVID-19 vaccines will need protection to prevent theft from the supply chain and to stop copying, substitution or adulteration that not only threaten patient health but also threaten the manufacturers’ finances and reputations.
Quality control and assurance tend to be well-established and well-resourced processes in major pharma companies. For every five people involved in production, one will be in quality management.
Covert security features and overt features on packaging, such as 2D barcodes and holographics, can help ensure integrity. A vial’s barcode could enable it to be traced to a particular batch made on a particular day, with records and release data to verify this. Aggregated packs of vaccines, transported by lorry, will be fitted with radio-frequency identification devices to convey not just consignment data such as temperature and vertical orientation, but also where the vehicle is. This enables checks on deviation and disturbance.
A marathon, not a sprint
Despite the effort going into developing and producing effective vaccines, it’s possible their safe shelf life could prove too short. Many biological preparations become unstable during storage, so typical vaccine development will involve taking product from the first production and putting it on stability trials for two years. With compressed timelines, these trials are taking place in parallel with other activities. Early samples are being monitored for degradation, while accelerated stability trials involve storing the product in variable formats with variations in temperature and humidity. Another critical engineering challenge for manufacturers is to generate and maintain these atmospheric conditions but, as Dr Cronin notes, “it’s only from this sort of work that we will get shelf-life data”.
As the global community reacts at an unprecedented pace to this challenge, Dr Cronin commends companies and academic institutions for the way they are working at speed and forming creative collaborations to pursue the discovery, development and manufacture of vaccines – despite having no guarantee of success. “It is testament to the leadership of these organisations and the professionalism and ingenuity of their scientists and engineers.”
***
This article was printed on 30 November. Since then, the Pfizer/BioNTech vaccine has been approved for use in the UK. Statistics quoted were correct at the time of printing.
The editors would like to thank Dr Rosie Dobbs for her help in putting together the abstract for this article.
This article has been adapted from "Developing fast-track COVID-19 vaccines", which originally appeared in the print edition of Ingenia 85 (December 2020).
Contributors
Rachel Jones
Author
Dr Donal Cronin FREng did a PhD in Chemical Engineering at the University of Birmingham and is an engineering and safety consultant to several leading pharmaceutical, bio-pharmaceutical, and chemical companies in Europe, Asia and North America. He has held a wide range of leadership roles in process development, operations and engineering. Most recently he was Head of Global Engineering and Technology in AstraZeneca.
Martin Smith did a PhD in Biochemical Engineering at UCL and is the Managing Director and Lead Consultant for Cawsam BioProcess Consulting Ltd. At the time of writing he was Head of Manufacturing Sciences and Technology at the Vaccines Manufacturing and Innovation Centre.
Chris Lucas did a masters in chemical engineering at Loughborough University. He is the Program Director European Programs for Janssen Vaccines and Project Director for Ashlie & Hall. At the time of writing he was the Chief Operating Officer at the Vaccines Manufacturing and Innovation Centre.
Keep up-to-date with Ingenia for free
SubscribeRelated content
Health & medical

Kidney dialysis
Small haemodialysis machines have been developed that will allow more people to treat themselves at home. The SC+ system that has been developed is lighter, smaller and easier to use than existing machines.

Engineering polymath wins major award
The 2015 Queen Elizabeth Prize for Engineering has been awarded to the ground-breaking chemical engineer Dr Robert Langer FREng for his revolutionary advances and leadership in engineering at the interface between chemistry and medicine.
Blast mitigation and injury treatment
The Royal British Legion Centre for Blast Injury Studies is a world-renowned research facility based at Imperial College London. Its director, Professor Anthony Bull FREng, explains how a multidisciplinary team is helping protect, treat and rehabilitate people who are exposed to explosive forces.
Targeting cancers with magnetism
Cambridge-based Endomag has helped treat more than 6,000 breast cancer patients across 20 countries. The MacRobert finalist uses magnetic fields to power diagnostic and therapeutic devices. Find about the challenges that surround the development and acceptance of medical innovations.
Other content from Ingenia
Quick read

- Environment & sustainability
- Opinion
A young engineer’s perspective on the good, the bad and the ugly of COP27

- Environment & sustainability
- Issue 95
How do we pay for net zero technologies?
Quick read

- Transport
- Mechanical
- How I got here
Electrifying trains and STEMAZING outreach

- Civil & structural
- Environment & sustainability
- Issue 95