Could organ-on-a-chip technology replace animal testing?
Did you know?
🧫 Organs in space + a potential replacement for animal testing?
- Organ-on-a-chip technology is used by pharmaceutical companies to test drugs and could reduce the need for animal testing
- To make miniaturised organ models with cell-sized features, bioengineers adapted an electronics manufacturing technique called photolithography
- Organs-on-chips are being used in space to investigate microgravity’s effect on the brain, and model a blinking eye and breathing lung
Drug development is painstakingly slow and plagued by failure: 90% of drugs that enter clinical trials in patients don't make it to the market.
To get to this point, the established pharmaceutical testbeds acting as analogies for the body are at best lab animals like mice, differentiated from humans by 60 to 80 million years of evolution; or at worst, featureless plastic plates, where cells are grown in a static solution of nutrients. These are a poor substitute for the 78 organs and over 30 trillion cells of numerous different kinds (not including bacteria).
That's to say nothing of the ethical implications of testing on animals; the serious side effects patients taking part may experience; or the fact that estimates for the typical cost of bringing a new drug to market range from hundreds of millions of dollars to over a billion.
Enter the organ-on-a-chip. In this emerging field, manufacturing techniques originally designed to pattern circuits and direct electrons in computer chips are being used to create cell culture systems with micrometre-scale architecture to guide cell growth and provide an environment that more closely mimics the body. These new devices aim to improve the drug discovery process and its safety for patients, and to reduce the use of animals in research.

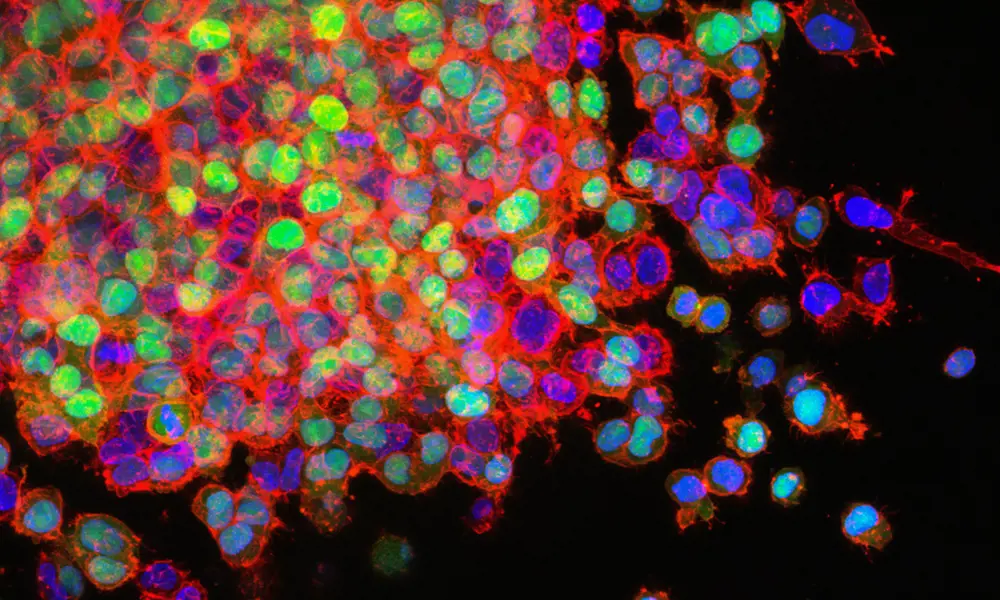
Tissue engineering requires human cell lines to be grown in special culture media © Drew Hays / Unsplash
Of cells, mice and men
Cells grown in a dish allow rapid, large-scale testing in a simplified system, well-suited for the fail fast, fail often approach: find out what doesn't work and move on to the next drug quickly before costs escalate. Pharmaceutical companies can test thousands to millions of drug candidates on cells this way.
But cells grown in traditional cell culture often fail to reflect the conditions found in the human body. Many biological experiments are conducted on cells that are inbred, diabetic couch potatoes who sit on a stiff plastic couch without exercise, gorge themselves on sugar once a day, live in their own excrement, and talk only to cells of like mind, says organ-on-a-chip specialist Professor John Wikswo, the Founding Director of the Vanderbilt Institute for Integrative Biosystems Research and Education. The implications of this discrepancy could mean failing a drug that could have worked, or failing to fail a drug and missing what could be a serious side effect.
After the initial drug screening, compounds are tested in animals. Studying how drugs react in the complex environment of a living body can provide crucial information to assess a drugs efficacy and potential toxicity. However, this information comes at the expense of animals, with over a million experimental procedures performed on animals in 2020 in the UK alone.
But even putting ethical issues aside, there are still fundamental differences between animals and humans that make animals flawed models for drug testing. While there are a surprising number of similarities across species, not all drug targets are the same. For example, the virus that causes COVID-19 enters a persons cells via a protein on the cell surface called the ACE2- receptor. While mouse cells also have this receptor, small molecular differences mean that the virus doesn't infect mice in the same way. New ways to test drugs in human cells that better resemble tissues and organs are therefore needed.
Many biological experiments are conducted on cells that are inbred, diabetic couch potatoes who sit on a stiff plastic couch without exercise, gorge themselves on sugar once a day, live in their own excrement, and talk only to cells of like mind.
Professor John Wikswo, Founding Director of the Vanderbilt Institute for Integrative Biosystems Research and Education
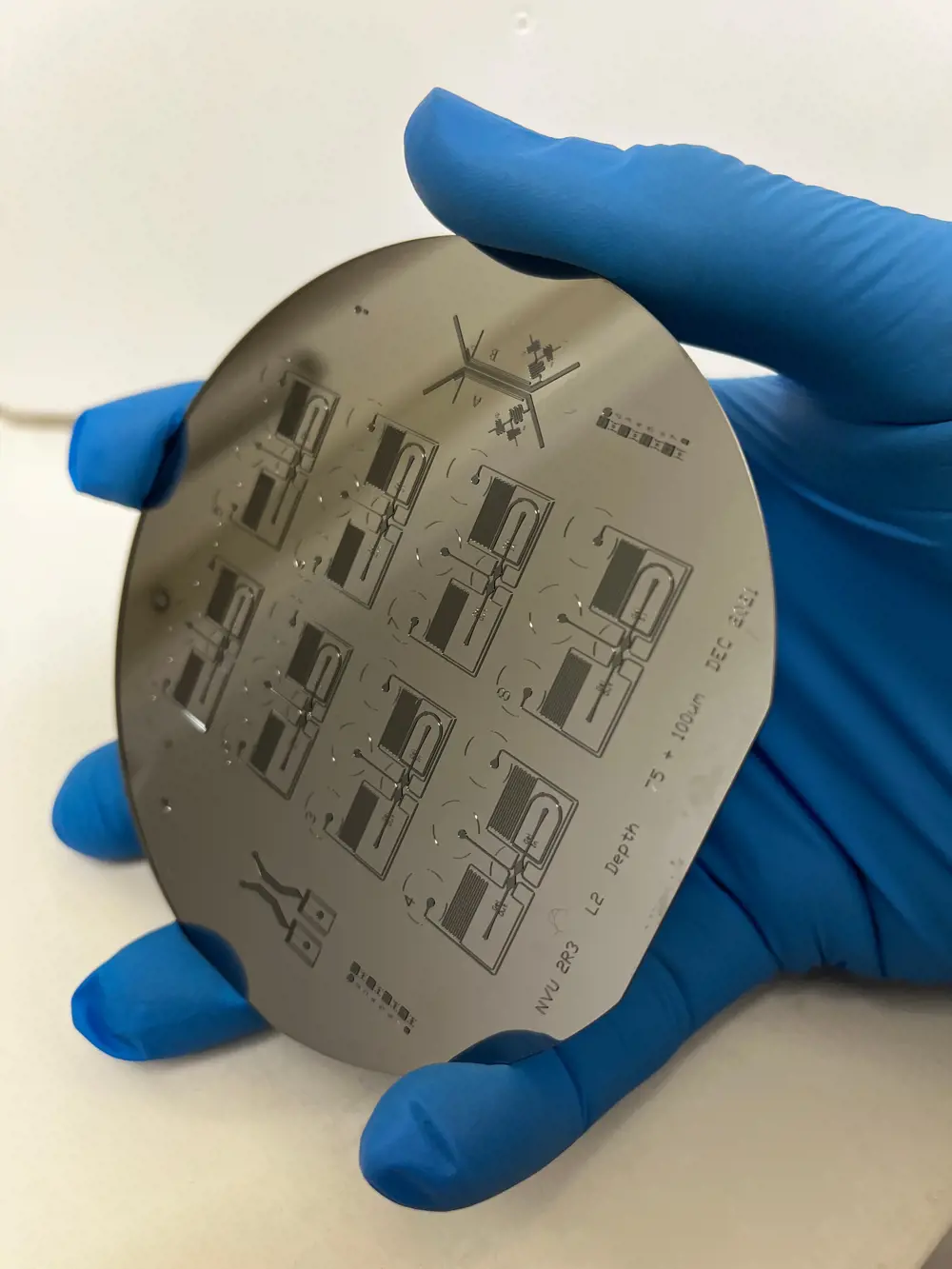
A silicon wafer held to the light reveals the micro-patterns that will be imprinted into a soft polymer to become the channels and cavities that will house human cells
From computer chips to organ chips
Cells have been shaped by evolution, but also interact with and respond to environmental cues. Some cues are chemical, such as hormones, signalling molecules and nutrients; some are physical, such as extension and compression during movement, or the stiffness of the tissue they grow in. Remove a cell from the body and it quickly changes its behaviour. Without the continuous flow and specific chemical environment of the bloodstream, for instance, blood vessel cells grown in a dish would quickly stop behaving like blood vessel cells. For cells to behave as they do in the body, these mechanical and chemical cues need to be mimicked to create a microenvironment that is more like the original organ or tissue, which helps to structure, guide and shape living cells. All this requires creating cell-scale architecture.
A typical human cell is just 10 micrometres wide – about a tenth of the width of a human hair. To create architecture on this minute scale, bioengineers adapted a technique called photolithography, normally reserved for patterning transistors onto silicon microchips. A raised design made using this technique on a silicon wafer can be used as a mould for a soft, rubbery polymer called polydimethylsiloxane (PDMS), which has the added advantage of being low cost and biocompatible, meaning that it is a good fit for growing cells.
Imprinting PDMS with tiny features and sealing it to a glass slide forms channels that can direct fluids and precisely position cells, creating a microfluidic device. By loading this device with the right cell types and chemical cues, bioengineers can begin to create an organ-on-a-chip. However, microfluidics differs from traditional fluid dynamics. Most engineers working with fluid dynamics must deal with the complex physics of turbulent flow, whether in hydroelectric dams or a jet engine.
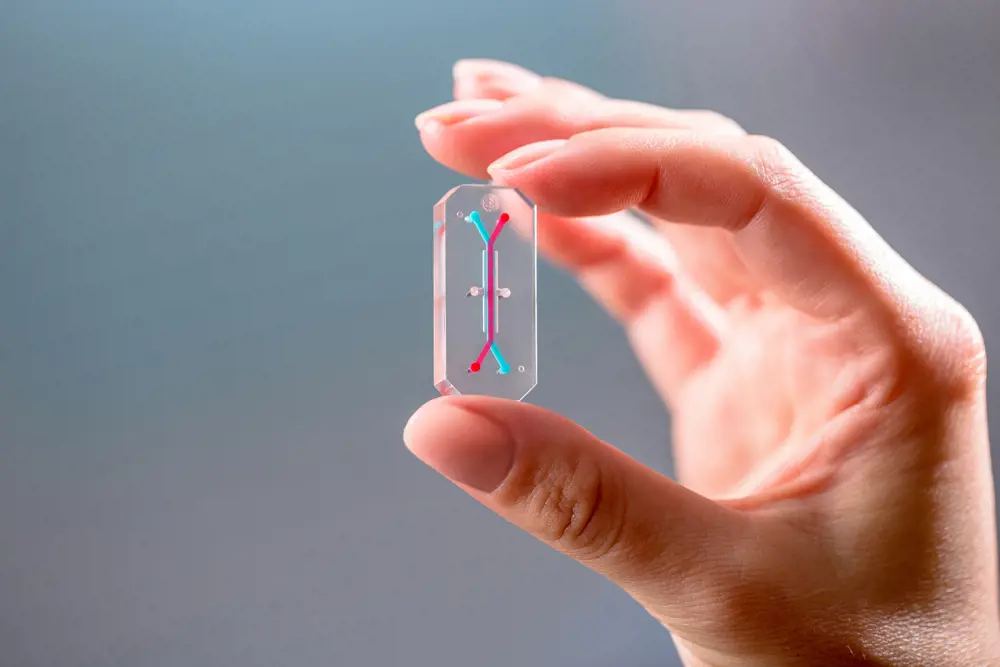
An organ-on-a-chip device designed by Wyss Institute spinout Emulate bIo. Cells can be grown in its microchannels, which are visualised with red and blue dyes © Emulate, Inc
At the microscale however, the physics is very different. In small channels, the relative importance of viscous (thick, sticky) forces prevails as inertia lessens. This means certain problems are simplified: flows are predictable and can be treated much like an electric circuit, making design relatively straightforward.
Meanwhile, other problems require a different approach altogether. For instance, imagine swimming in honey. Getting anywhere is difficult because the fluid is so viscous, and the inertia of each stroke is not enough to propel you forward. At the microscale, water behaves more like honey. Biology has adapted to this by using different ways of swimming, such as the corkscrew tail of a sperm or a bacterium. Engineers working on microfluidics are now exploiting some of the quirks of physics in microscale channels to control flows of chemicals with high temporal and spatial precision, not only for organs-on-chips, but also for chemical reactions and 'lab-on-chip' applications.
Evidently, all this work requires cross-disciplinary interactions between engineers, biologists, physicists, and chemists. In the UK, the Organ-on-a-Chip Technologies Network is helping researchers do exactly this, providing a UK-based platform to help advance organon- a-chip technologies and improve drug discovery.
Pharma companies are playing a key role, with GlaxoSmithKline putting TARA Biosystems' beating heart-on-a-chip through its paces and AstraZeneca testing the waters with Emulate Bio's kidney-on-chip. Further afield, Emulate's chips are also being used in high-profile collaborations with Roche and Johnson & Johnson.
Researchers are also using these different organ-mimicking devices to provide new insights that will help them better understand human disease and discover new potential treatments. From neuronal circuits to study Alzheimer's disease, to a breathing lung-on- a-chip to study COVID-19, researchers are using organs-on-chips to take a closer look at how disease can affect an organ. Fundamental biology is covered too: Emulate is due to send its organ-on-a-chip technology to the International Space Station to study how the brain works under microgravity.
Emulate is due to send its organ-on-a-chip technology to the International Space Station to study how the brain works under microgravity.
Living neural circuits
A celebrated quote often attributed to Einstein says "everything should be made as simple as possible, but not simpler". Working towards this principle, an organ-on-a-chip does not attempt to completely replicate a whole organ, but instead simplifies it to investigate specific aspects of its function in a context relevant to the question. This could be structural, such as physically guiding neurons' growth. It could be mechanical, such as recreating the flow of blood through a vessel, the breathing motion of the lung or the physical stiffness of the bone. Or it could be chemical and electrical cues, via the signals between different cells.
One example of how microfluidic technologies can distil a very complex organ to a simplified experimental tool is in the brain's neuronal circuits. When neurons are grown in a dish, they do not form the organised circuits we see in the brain on their own. To encourage the creation of simplistic living neuronal circuits, researchers have used organ-on-a-chip devices like the XonaChip® to guide connections between cells in different compartments. Here, filaments called axons protrude from neurons and grow along microchannels smaller than the cells themselves. They link together multiple cell culture compartments to form simplistic neuronal circuits, which can even be integrated with electrical sensors to monitor neuronal function.
Of course, these simplified circuits are a far cry from the complexities of the human brain. While the human brain has about 100 billion neurons, devices like the XonaChip® typically contain around a thousand cells, around a thousand times less than a fly brain.
Nevertheless, these systems can be used to simplify complex problems, such as how brain disorders like Alzheimer's disease develop and spread through the brain. Neuronal dysfunction in Alzheimer's is caused by accumulation of a defective form of a protein called tau. Using neuronal-circuits-on-a-chip, research groups at the University of Southampton and the University of Strathclyde have independently been able to track how this defective tau protein can spread through neuronal connections to 'infect' otherwise healthy cells. By enabling new insights into the mechanisms of how this protein spreads between neurons, organ-on-a-chip technologies could help reveal new ways to stop Alzheimer's disease in its tracks.
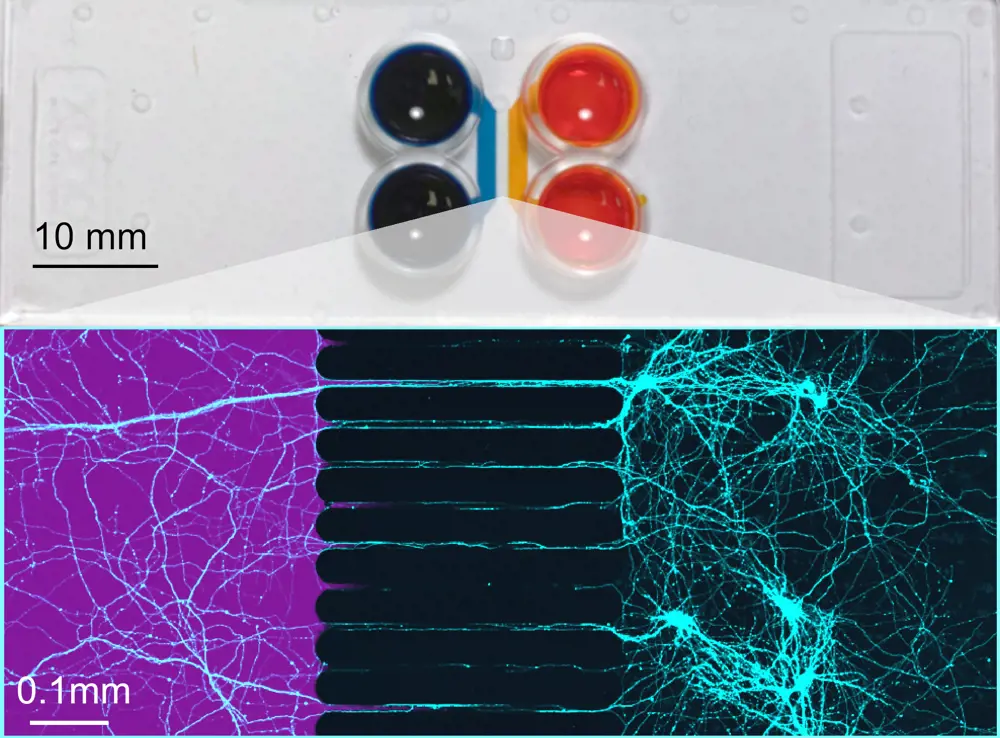
Top: A two compartment Xona microfluidic chip, with cell culture chambers in blue and orange. Bottom: Microscale channels are used to guide connections between axons (coloured in teal) between compartments. The high fluidic resistance of these channels means that a treatment (here a purple dye) can be given to one part of the circuit without directly affecting the other © Xona Microfluidics, Inc
Breathing life into organs-on-a-chip
Organs, of course, do not just consist of one cell type. While patterning single cell types allows new insights into how they function, the real power of organ-on-a-chip technology comes when multiple cell types are cultured together. The first organ-on-a-chip to do this was Emulate's lung-on-a-chip. Zooming in to a cross-section of that device (above), you can see how epithelial cells that usually line the alveoli of the lung were grown on a membrane above a channel coated with blood vessel cells. The upper channel was exposed to air, while in the lower channel, a blood-mimicking media was flowed through. Vacuum channels that flank the central cell culture chambers were then used to stretch the rubbery device and mimic the cyclic mechanical strain caused by breathing. Remarkably, cells responded strongly to this breathing motion. The barrier formed by the epithelial cells became stronger, and cilia (the hairs) on the cells began moving mucus and clearing debris more effectively, modelling how the lungs remove contaminants.
Growing multiple cell types together also allows researchers to study how they interact. Professor Daniel Pennington, an immunologist at Queen Mary, University of London (QMUL), is now using these lung-on-a-chip systems to study how COVID-19 can not only affect lung cells but also cause blood vessel dysfunction. By comparing the effects of plasma from severe COVID-19 patients and healthy controls in the chip, the researchers will be able to study how interactions between blood vessel cells and lung cells can be disrupted. With a few tweaks and by populating it with different cell types, the same device can be used to mimic different organs and diseases. In fact, QMUL has recently established an organ-on-a-chip research centre in partnership with Emulate, using its chip technology to study organs from kidney to colon. Elsewhere, Swiss biotech company AlveoliX, Delft-based BI/OND and Cambridge's CN Bio have been developing their own microengineering approaches to construct other types of organ models, from circulation in blood vessels to oxygen gradients in the liver.
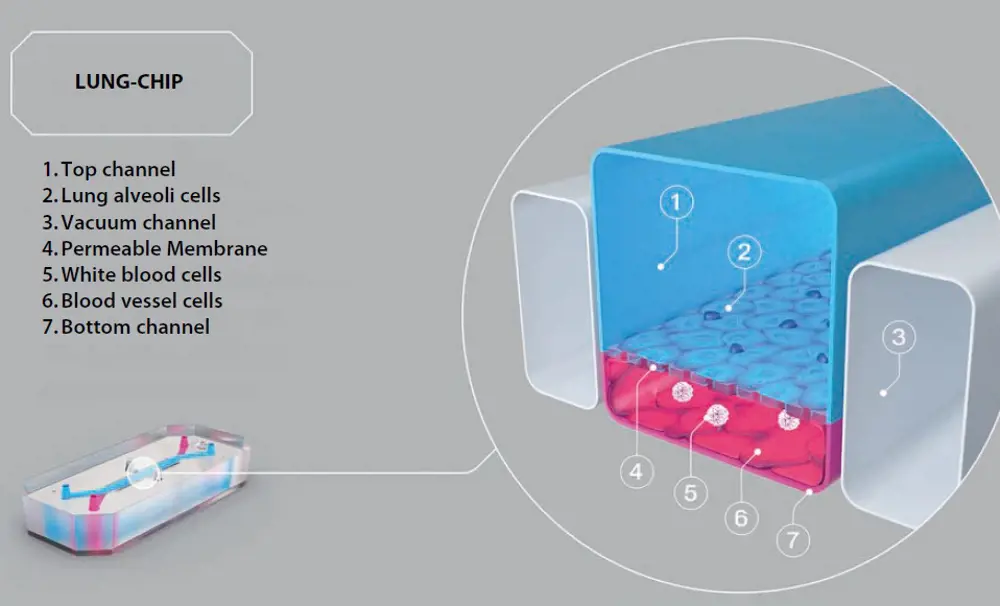
Emulate’s lung-on-a-chip allows the lung’s mechanical breathing motion to be recreated using vacuum channels © Emulate, Inc
Other researchers are building their own custom chips in-house. At Brunel University, a team of life sciences and engineering experts, led by mechanical engineer Dr Ruth Mackay and toxicologist Dr Elisabete Silva, are developing models exclusively focused on women's health. Four main organ-on-a-chip models replicating the breast, vagina, ovary, and placenta will primarily be used to study cancer and bacterial infections. To allow them to produce custom 3D micro-environments that are optimised for specific cell types, the team is incorporating bioengineering techniques such as electrospinning (using electric charges to spin polymer fibres) and 3D printing, alongside soft lithography.
Looking to the future, if new technologies can be developed that allow patient-specific cells to be used in these models, it could mean that eventually, drugs could be tested on a 'mini-me' on-chip before they ever reach a patient.
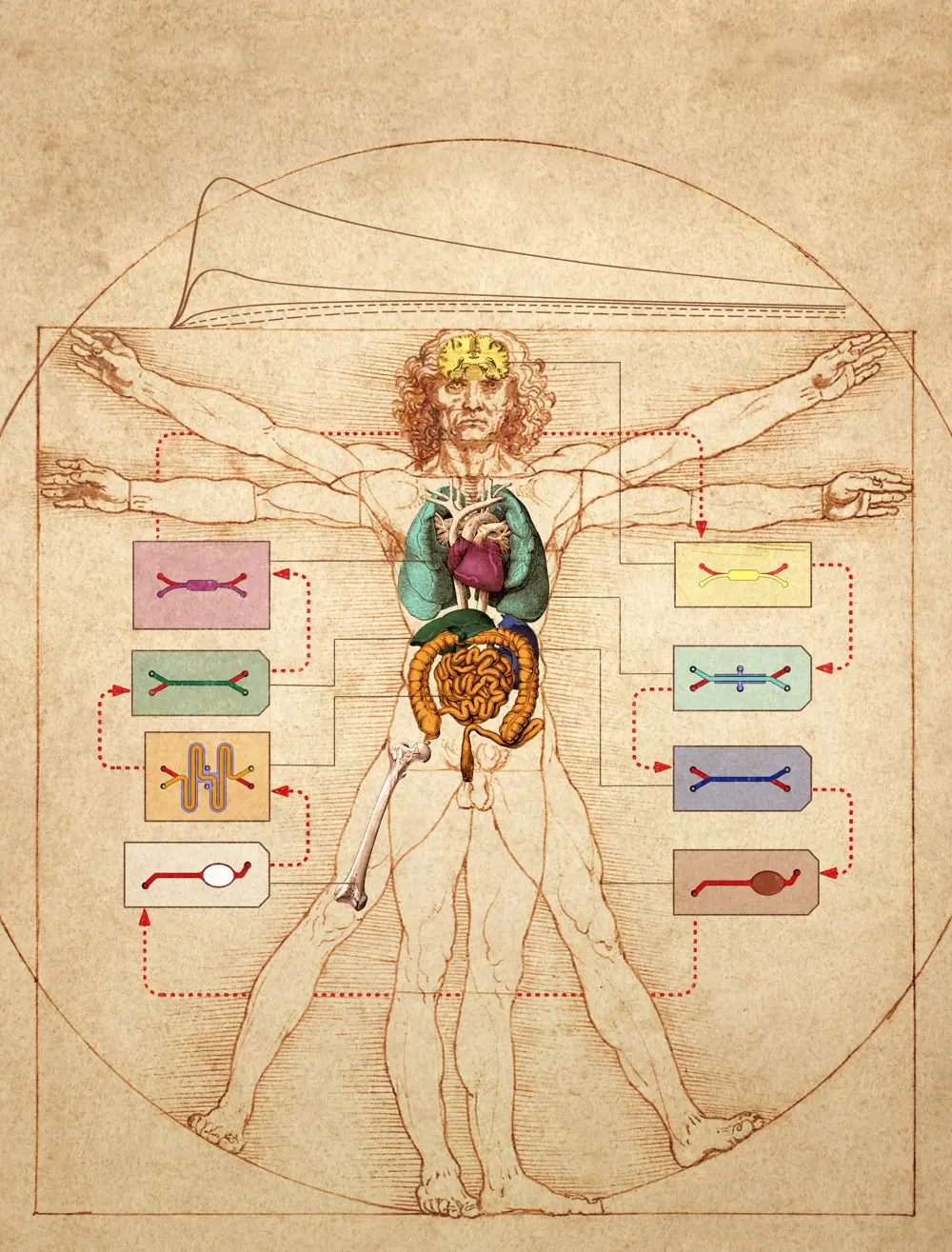
Linking multiple organ-on-a-chip devices could be used to test drug absorption, distribution, metabolism, excretion, and efficacy in ‘body-on-chip’ studies © Wyss Institute at Harvard University
Body-on-a-chip
The use of organs-on-chips by pharmaceutical companies like Johnson & Johnson and Pfizer to evaluate candidate drugs is a huge step forward from cells in a Petri dish. But drugs don't just affect one organ, they affect the whole body. A drug meant for the heart might be taken up through the gut, metabolised by the liver, then travel through the bloodstream and ultimately cause a toxic side effect in the kidney. So, what about linking chips together to make a body-on-a-chip?
It certainly poses a huge and complex challenge, but this hasn't stopped researchers pushing the boundaries. In 2018, CN Bio and the Massachusetts Institute of Technology (MIT) successfully linked 10 organs-on-chips together, each consisting of over a million cells. In 2020, the Wyss Institute reached the same milestone at the end of an eight-year, $37 million project. This work also integrated new mathematical and computational modelling techniques to allow analysis and interpretation of how these readouts scale in relation to our own, complex, life-sized human bodies. Looking to the future, if new technologies can be developed that allow patient-specific cells to be used in these models, it could mean that eventually, drugs could be tested on a 'mini-me' on-chip before they ever reach a patient.
***
This article has been adapted from "Life on a chip", which originally appeared in the print edition of Ingenia 90 (March 2022).
Contributors
Dr Paul Holloway uses microfluidic technologies to precisely define cellular micro-environments and enable novel insights into neurovascular biology. He is a member of the Organ-on-a-Chip Technologies Network.
Keep up-to-date with Ingenia for free
SubscribeRelated content
Health & medical
A gamechanger in retinal scanning
2006 MacRobert Award winner Optos rapidly became a leading medical technology company and its scanners have taken millions of retinal images worldwide. There is even a display at the Science Museum featuring the Optos development. Alastair Atkinson, of the award-winning team, describes the personal tragedy that was the trigger for the creation of Optos.

Kidney dialysis
Small haemodialysis machines have been developed that will allow more people to treat themselves at home. The SC+ system that has been developed is lighter, smaller and easier to use than existing machines.

Engineering polymath wins major award
The 2015 Queen Elizabeth Prize for Engineering has been awarded to the ground-breaking chemical engineer Dr Robert Langer FREng for his revolutionary advances and leadership in engineering at the interface between chemistry and medicine.
Blast mitigation and injury treatment
The Royal British Legion Centre for Blast Injury Studies is a world-renowned research facility based at Imperial College London. Its director, Professor Anthony Bull FREng, explains how a multidisciplinary team is helping protect, treat and rehabilitate people who are exposed to explosive forces.
Other content from Ingenia
Quick read

- Environment & sustainability
- Opinion
A young engineer’s perspective on the good, the bad and the ugly of COP27

- Environment & sustainability
- Issue 95
How do we pay for net zero technologies?
Quick read

- Transport
- Mechanical
- How I got here
Electrifying trains and STEMAZING outreach

- Civil & structural
- Environment & sustainability
- Issue 95