
Engineering polymath wins major award
That the winner of an award as prestigious as the Queen Elizabeth Prize for Engineering (QEPrize) should be based at a world-class academic organisation like the Massachusetts Institute of Technology (MIT) is not surprising. Less predictable, at least to anyone unfamiliar with the career of chemical engineer Dr Robert Langer, is the sector of MIT with which he is closely affiliated: the Koch Institute for Integrative Cancer Research. How does a chemical engineer come to be working at the highest level inside a medical research organisation?
Part of the answer can be found in the nature of the Koch Institute itself. Set up with the aim of putting biologists, chemists and clinicians together with computer specialists, materials scientists and engineers, it seeks to bring an interdisciplinary approach to cancer research. His laboratory at MIT – with over 100 students, postdoctoral students, and visiting scientists at any one time – is the world’s largest academic biomedical engineering laboratory. He has over 1,000 issued and pending patents, over 200 major prizes to his name, and is the most cited engineer in history – as reported by Science magazine.
Langer’s interests, principally in biomedicine, are bewilderingly broad. They have ranged from a chemotherapy wafer for treating brain cancer to a new kind of surgical adhesive inspired by geckos and their ability to walk up walls. He has also proven adept at devising solutions to problems and then overseeing their passage out of the laboratory and into commercial production. But it is in two areas of work close to the mainstream of biomedical research that his reputation has been built: the controlled release of drugs; and the development of tissue engineering.
Langer’s position is a reflection of the man himself: his urge to apply his innovative skills and imagination to a variety of problems that include, but go beyond, those conventionally thought of as the province of the chemical engineer. When he left Cornell University in 1970, he says that he looked for something that would interest and excite him and found inspiration in the work of Judah Folkman, a Harvard professor and surgeon at Boston Children’s Hospital.
I was the only engineer in the department and I could see all sorts of problems for which engineering had solutions
Dr Robert Langer
“He was doing research on angiogenesis, the formation of new blood vessels. His clinical aim was to find a drug to block it, and from that develop a new way of preventing the growth of tumours. He was a very charismatic man, and because he was following an unorthodox line of research, he was prepared to consider people with different backgrounds. I thought what he was doing was very compelling, and he was very compelling.
“I was hired to find something that would inhibit blood vessel formation, and so halt tumour growth. We succeeded and published the findings in Science in 1976. It was probably the first example of successful antiangiogenesis. Biochemists and pharmacologists had tried to solve the problem, but not a chemical engineer. I was the only engineer in the department and I could see all sorts of problems for which engineering had solutions.”
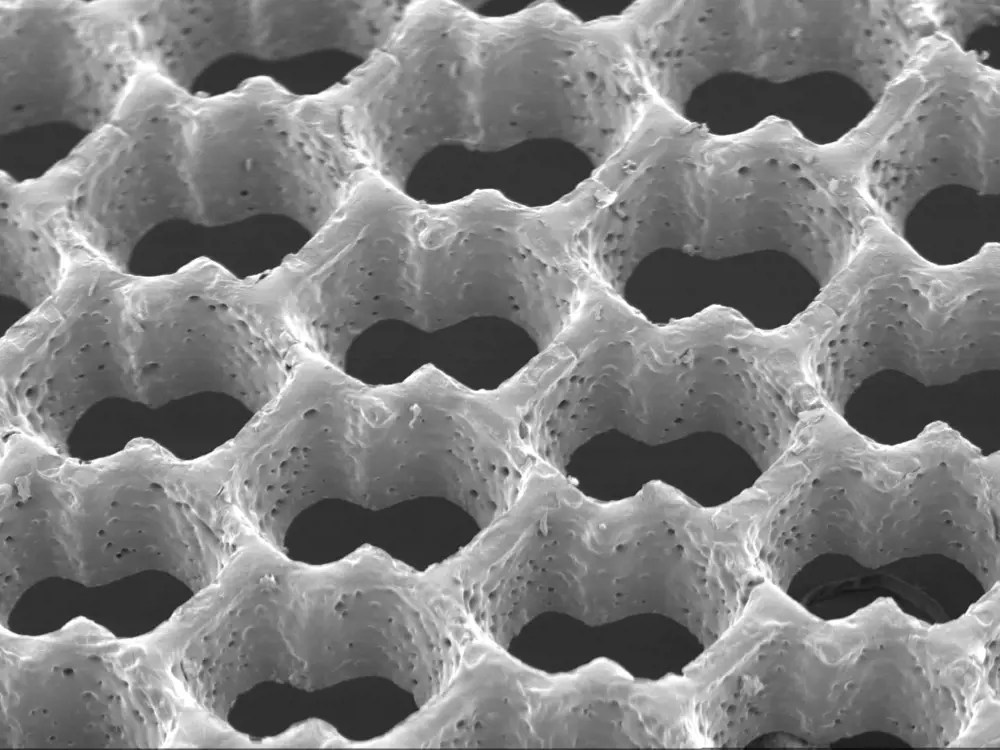
Scanning electron micrograph of an accordion-like honeycomb scaffold for cardiac tissue engineering. Original magnification =100x © GC Engelmayr Jr (MIT)
Controlled release technology
Another of the goals that Langer and Folkman had been pursuing was the development of a method for the slow and controlled release of drugs inside the body. Swallowing a pill by mouth two or three times a day or being given an injection are not always ideal methods of delivery. Even if the drug is given at the right time, its level in the patient’s body will inevitably fluctuate around the optimum dose: first too high; then, as it is removed from the body, too low. Controlled release can smooth out these fluctuations. Over a longer period, it may also relieve the patient of the need to remember to take a medicine at set intervals. Diseases including some cancers and mental illnesses are now treated by this controlled-release means. But Langer’s success did not come overnight.
“In Judah Folkman’s lab, I experimented with different approaches to the controlled release of drugs, but using a polymer to store and release them seemed the best and simplest. Polymers are inert, and were already being used in medicine. Folkman and I had got some existing polymers from other researchers – but even these people didn’t think they would work as a drug delivery system. It was known that you could deliver small, lipophilic molecules from polymers, but not large molecules of the size we wanted to use. People were very sceptical.
“Patent examiners were not impressed either. Nor were the drug industry or research grant awarding bodies like the National Institutes of Health. It took many years before controlled release was used routinely in medicine. We were up against innate conservatism, and also professional wisdom that held it just wasn’t going to work. It was very discouraging. In the end, the technology was accepted, but it took 12 to 15 years. Moving ideas from chemistry and engineering into medicine does sometimes take time.”
Once he had discovered how to create polymer micro- and nano-particles that could support and release sensitive protein-based drugs in the body, he used this technique to test possible drugs to control angiogenesis. He and Dr Folkman isolated the first substances that blocked angiogenesis; such substances have been used to treat over 20 million patients.
An early application of the controlled-release technology was in polymer microspheres that deliver nanopeptide drugs over several months and are now widely used to treat prostate cancer and endometriosis. Similar approaches have led to new treatments for schizophrenia, alcoholism and drug addiction.
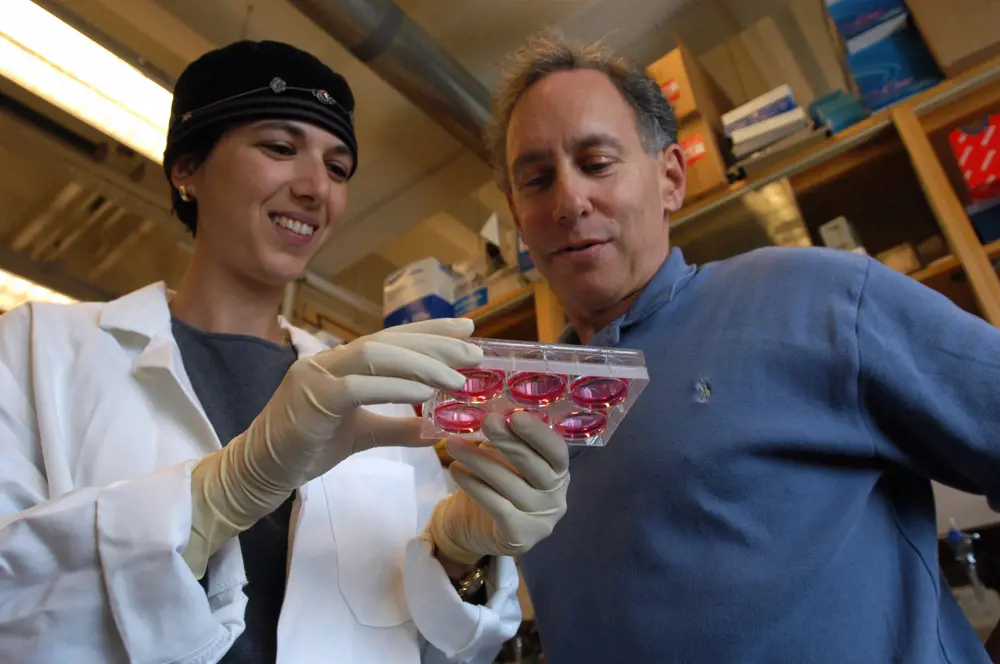
Dr Shulamit Levenberg with Dr Robert Langer in 2003. The MIT engineers ‘seeded’ human embryonic stem cells, which have the potential to differentiate into a variety of specialised cells, onto a biodegradable polymer scaffold. By treating the scaffold/stem cell structure with chemical cues, or growth factors, known to stimulate the formation of specific cell types, the researchers coaxed the stem cells to form tissues with characteristics of developing human cartilage, liver, nerves and blood vessels
Tissue engineering
Langer also has been influential in the field of tissue engineering, the process of growing tissues or even whole organs outside the body before implanting them in patients who have suffered injury or disease. Langer was among the pioneers of this enterprise. Although research has been in progress for many years, it remains a technique yet to be fully exploited. If it lives up to expectations, it will ultimately make a significant impact on medicine.
“I got involved in tissue engineering in 1983, when Jay Vacanti, a surgeon colleague of mine who ran the liver transplant programme at Boston Children’s Hospital, started asking if we could develop tissues or organs for small babies who were dying of liver failure. The basic idea is to create a three-dimensional framework to which living cells would be able to attach themselves, and then grow and divide to produce something that could be implanted in the patient and take over the role of whatever tissue or organ had been lost. We had to start by finding the right material for the framework and then work out how to configure it to provide a substrate on which cells would grow. We also had to design reactors or incubators to create an environment in which the tissues would grow.
Dr Shulamit Levenberg with Dr Robert Langer in 2003. The MIT engineers ‘seeded’ human embryonic stem cells, which have the potential to differentiate into a variety of specialised cells, onto a biodegradable polymer scaffold. By treating the scaffold/stem cell structure with chemical cues, or growth factors, known to stimulate the formation of specific cell types, the researchers coaxed the stem cells to form tissues with characteristics of developing human cartilage, liver, nerves and blood vessels
“We started with polylactic acid and co-polymers and other materials that already had FDA approval for medical use. We wanted materials that were biodegradable, that lasted long enough for cells to grow and make their own supporting matrix, but would not last forever. The insoluble polymers we use break down to form more soluble monomers. We began using polyanhydrides, a family of materials originally developed for the artificial fibre industry, but rejected because of their relative instability. We’re now trying to understand how different materials and different stresses affect stem cell growth, and we’re developing all kinds of new tissue substitutes from intestines to vocal cords.”
Vacanti and Langer’s research has paved the way for major innovations in tissue engineering, pioneering synthetic polymers that could deliver cells to form specific tissue structures. This concept led to the development of a new kind of artificial skin, now approved by the FDA for use on burn victims and patients with diabetic skin ulcers. Many other such systems, including ones for new cartilage formation and spinal cord repair, are now in clinical trials.
Swallowing the needle
💊 The useful drug delivery system invented by Langer and his team
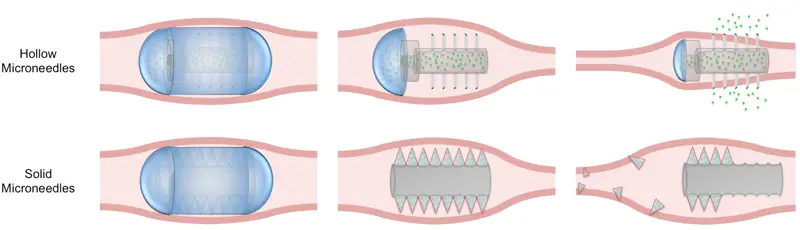
Because they are poorly absorbed from the gut or even destroyed in it, many of the therapeutic products of the medical biotech industry cannot be taken by mouth. They must be given by injection: less popular with patients and less convenient. Langer and his colleagues have invented a method of using microneedles to deliver drugs through the lining of the gut.
Each device comprises a set of tiny needles, housed within a capsule small enough to swallow and made of a material that will dissolve at the desired location in the gut. Each needle is primed with the drug to be delivered. As the needle points come into contact with the gut lining, and penetrate it, the drug enters the patient’s circulation. The absence of pain receptors in this region of the body means that the patient feels nothing.
The empty needles are free to pass on down and eventually out of the body Even at this stage of the needles’ journey, the patient should feel nothing; surgeons have discovered that sharp objects much larger than these tiny needles can be voided from the gut without causing discomfort.
The system has been tested using insulin in experimental animals. These showed the predicted changes in their blood sugar levels, and the researchers could find no evidence of tissue damage. The system might be suitable for delivering vaccines as well as drugs.
Getting new ideas to market
A great deal of medical research is bedevilled by failures of translation: the movement of a product or an invention out of the laboratory and into the clinic for everyday use. Langer has been notably successful in tackling this. He has helped generate more than 1,000 patents and he, with colleagues, has already spun out some 27 companies, and the process continues – see Swallowing the needle. When he started creating new companies to exploit laboratory findings, it was still relatively new territory for most academics. Nowadays, if not exactly routine, the commercialisation of novel products and devices is no longer unfamiliar. More and more academics, often with the encouragement of their universities, are doing it.
“It’s important to me personally to see the work that we do gets out to the world. When a company has been formed, I go on taking an interest in it, usually by being on the scientific advisory board or the board of directors. I’m there for advice as it’s needed. I don’t much enjoy the financial parts of commercialising something, but I do enjoy the brainstorming and working with the creative people in the company. It’s very satisfying when something that five or 10 years ago was just an idea in your head gets into the clinic.
“I have a couple of hundred ex-students who are now senior academics around the world, and they’ve taken these ideas on board. The university plays a significant role in the process because the patents are licensed through it, and part of any profit comes back to MIT. I wouldn’t say I encourage all my students and colleagues to set up companies, but I do encourage them to do what they want. I’d say no more than 15% of my time is taken up by these commercial activities. There are ex-students of mine working in almost all of these companies, so for me it’s a way of being able to maintain the relationship I’ve had with them.”
One of Dr Langer’s most recent projects is a microchipbased implant that can store and release precise doses of a drug on demand or at scheduled intervals for up to 16 years. Microchips Biotech, the company he co-founded to commercialise the development, announced in December 2014 that it has completed clinical demonstration. Unlike traditional drug delivery platforms, Microchips Biotech’s implant can respond to wireless signals, which can activate, deactivate, or modify the frequency or dose of the drug, without being removed from the patient.
The company is looking initially at three areas for such an implant: diabetes, female contraception, and osteoporosis, which all require regular, longterm dosage. The contraceptive approach is funded by the Gates Foundation, as are new ways of providing single-step immunisations for polio and other vaccines, providing longacting malaria drugs, and providing essential minerals. Dr Langer’s lab is currently pursuing all of these new techniques.
Raising engineering's profile
👑 What is the Queen Elizabeth prize and the design the trophy competition?
The Queen Elizabeth Prize for Engineering (QEPrize) is an international £1 million prize that rewards and celebrates the engineers responsible for a ground-breaking innovation that has been of global benefit to humanity.
The objective of the QEPrize is to raise the public profile of engineering and to inspire young people to become engineers. In the last year, a number of initiatives have been run to help change perceptions of engineering careers. The launch of nominations for the QEPrize in March 2014 coincided with a report that looked into parents’ attitudes to their daughters studying engineering. The findings of the report were then published online and led to numerous debates and interviews across various media platforms.
During the summer of 2014, a national competition was held for 16- to 24-year-olds to design the trophy that will be presented to the winner by HM The Queen. There were hundreds of entries from around the country and an engineering student from Glasgow, Euan Fairholm, won the competition, with his design, ‘The Golden Crown’.
A global ambassador network has also been set up by the QEPrize, comprising inspirational young engineering role models whose purpose is to show parents, teachers and young people the diversity and scope of engineering. The network was launched with an engineering party in 2014 which showcased some of the most innovative and creative examples of new technology.
By rewarding engineers who have changed the world, the organisers of the QEPrize hope to uncover their stories, and in the process, encourage young people to take up the challenges of the future. These aims resonate across the world and the messages are global: engineering is an exciting and fulfilling profession, engineers are at the heart of society and engineers create the future.

Euan Fairholm shows his winning trophy design ‘The Golden Crown’ to Lord Browne of Madingley FREng FRS
QE Prize
Announcing the award earlier this year, Lord Browne of Madingley FREng FRS, chair of the Queen Elizabeth Prize for Engineering Foundation, described Langer as an extraordinarily talented engineer with incredible determination and vision whose work has transformed the lives of people across the globe. Another of the judges, the physicist and TV presenter Professor Brian Cox, was impressed by the number of engineers supporting Langer’s nomination who said they were in the profession because of the inspirational teaching they had received from him. This resonated with the QEPrize organisers who work to both raise the public profile of engineering and to inspire young people to become engineers – see Raising engineering’s profile.

The 2015 QEPrize judging panel was chaired by Lord Alec Broers FREng FRS, seen here deliberating with fellow judge Professor Frances Arnold (California Institute of Technology)
Dr Langer will come to London again later in the year to formally receive the QEPrize trophy from HM The Queen at Buckingham Palace. His citation reads that through his rigorous approach to medical materials design and by pursuing commercial implementation of his innovations, Langer not only solved the problem of controlled release large molecule drug delivery, he fundamentally changed the way that materials are used in medicine. At a time when few engineers worked in experimental medical research, Dr Langer’s perspective on medicine was ground-breaking: he was confronted by clinical problems, but sought chemical and engineering solutions. It is estimated that the technologies produced by Dr Langer’s laboratory have helped improve the lives of two billion people worldwide.
***
This article has been adapted from "Engineering polymath wins major award", which originally appeared in the print edition of Ingenia 63 (June 2015).
Contributors
Geoff Watts
Author
Keep up-to-date with Ingenia for free
SubscribeRelated content
Health & medical

Kidney dialysis
Small haemodialysis machines have been developed that will allow more people to treat themselves at home. The SC+ system that has been developed is lighter, smaller and easier to use than existing machines.
Blast mitigation and injury treatment
The Royal British Legion Centre for Blast Injury Studies is a world-renowned research facility based at Imperial College London. Its director, Professor Anthony Bull FREng, explains how a multidisciplinary team is helping protect, treat and rehabilitate people who are exposed to explosive forces.
Targeting cancers with magnetism
Cambridge-based Endomag has helped treat more than 6,000 breast cancer patients across 20 countries. The MacRobert finalist uses magnetic fields to power diagnostic and therapeutic devices. Find about the challenges that surround the development and acceptance of medical innovations.
Transcranial magnetic stimulation
Magnetic stimulation, especially of specific regions of the brain, has added greatly to the understanding of normal brain function and is being increasingly used to stimulate nerves for therapy. Mike Polson, Engineering Director of Magstim Company Ltd, talks about the history and potential of transcranial magnetic stimulation.
Other content from Ingenia
Quick read

- Environment & sustainability
- Opinion
A young engineer’s perspective on the good, the bad and the ugly of COP27

- Environment & sustainability
- Issue 95
How do we pay for net zero technologies?
Quick read

- Transport
- Mechanical
- How I got here
Electrifying trains and STEMAZING outreach

- Civil & structural
- Environment & sustainability
- Issue 95