
Processing the plastic problem
In recent years, there has been a steady rise in public consciousness of the existence of waste plastic and the threats it poses. Images of plastic bags, bottles and pots dumped as landfill or, even more damning, floating in otherwise pristine oceans far from their original site of use have become ubiquitous. Plastic is now political and this development has led to increasing pressure to do something about a state of affairs that is universally agreed to be unacceptable. But what?
Suggested remedies have ranged from the technical (make more plastic biodegradable, for example) to the behavioural (using it less casually). Recycling is, of course, prominent among the technical solutions and much has already been done. Figures quoted by the British Plastics Federation suggest that the UK now has a 58% collection and recycling rate for plastic bottles, and 32% for pots, tubs and trays.
Most current recycling is by mechanical methods: chop up the plastic, wash it, melt it and reuse it. However, plastic is not all of a piece. As described in a previous Ingenia article on the recycling of household waste, the advent of near-infrared sorting techniques has boosted the efficiency with which different types of plastic can be identified and separated. These systems rely on shining infrared light onto a conveyer belt carrying a thinly distributed stream of plastic items. The peak wavelength of light reflected from each piece of plastic will depend on the category to which it belongs. A spectroscope mounted above the conveyor belt measures this wavelength for each item and records its position on the belt. At the end of the line, puffs of air blow each piece off the belt in a specified direction, and into a chosen collection vessel, according to its type.
it would be even more efficient to recycle plastic of all varieties and do so without first having to sort them. Achieving this goal requires chemical as opposed to mechanical methods
These systems are effective and have greatly boosted the efficiency of recycling, particularly for high-value polymer streams such as polyethylene terephthalate (PET) drinks bottles and high-density polyethylene (HDPE) milk bottles. After sorting, these are shredded, washed and re-extruded to form pellets ready for moulding applications. However, not all plastic is amenable to mechanical recycling methods. Clearly, it would be even more efficient to recycle plastic of all varieties and do so without first having to sort them. Achieving this goal requires chemical as opposed to mechanical methods. In principle – indeed in reality – it too can be done, but the task of implementing it on a commercial scale, and with an acceptable level of emissions, has not proved an easy one.
Plastic waste: The scale of the problem
🥤Plastic waste statistics and facts
Commonly used plastics are not biodegradable and simply accumulate in landfill sites or in the environment more generally. Between 1960 and 2005, the proportion of plastic in the municipal solid waste of middle- and high-income countries rose from 1% to 10%.
Most of the statistics about plastic waste are disheartening. Prior to 1980, the amount of plastic incinerated or recycled was negligible. As a result, around 60% of all the plastic ever made is still with us in the form of waste. Estimates of the quantity of plastic thrown or washed into the world’s oceans in just one year range from 4 to 12 million metric tonnes. Another estimate suggests that by 2050 the weight of plastic in the sea will exceed that of fish.
It has even been claimed that because plastic waste is now so ubiquitous, future geologists will be able to use it as a marker of the Anthropocene, the name given to our current geological era.

Plastic is an everyday item, used in everything from food packaging to toiletry products. In 2015, the UK alone generated 2.26 million tonnes of plastic packaging waste, according to government figures © Recycling Technologies
Chemical recycling
Chemical recycling relies on a process called pyrolysis: the thermal decomposition of materials at high temperature in an atmosphere free of oxygen. In this context, the heating process fractures the large organic molecules that make up plastic into smaller ones. ‘Thermal cracking’, as this breakdown is generally known, is already familiar as the cornerstone of petroleum refining. In the case of waste plastic, thermal cracking reverses the process by which the plastics being recycled were originally synthesised from smaller starting molecules.
Chemical recycling relies on a process called pyrolysis: the thermal decomposition of materials at high temperature in an atmosphere free of oxygen
Pyrolysis is not a new idea; in fact, it has been in use since Archimedes and is the basis of charcoal and town gas (a gaseous mixture, used as a fuel, that bituminous coal releases when burned). The very familiarity of the chemistry lying at the heart of the process prompts bewilderment over why chemical engineering companies are not already exploiting it to tackle the waste plastic problem. This in turn drops a hint that, in practice, the task of cracking plastic to make other reusable products is not as straightforward as it might seem.
A few companies, particularly in Asia, have tried chemical recycling. Not all have succeeded, but some are still in business. In Europe, Plastic Energy is a company with UK headquarters and two waste plastic plants in Spain. It is keen to build a plant in the UK, but this is still in the planning stage.
the task of cracking plastic to make other reusable products is not as straightforward as it might seem
Enterprises already in operation in the UK are thin on the ground. The newest entrant to the field is Oxford Sustainable Fuels (OSF), a company spun out of the University of Oxford’s Department of Chemistry that plans to reduce plastic’s environmental impact by providing a low-energy process to convert it and other solid hydrocarbon (an organic compound consisting entirely of hydrogen and carbon) waste material into high-quality transportation fuels. Right now, the most compelling evidence that Britain could be recycling mixed plastic into useful products is being provided by engineering company Recycling Technologies.
When the company’s engineers began developing its RT7000 machine they faced a variety of problems, several that they have solved empirically. However, purely engineering hurdles were not the only, or even the toughest, that the engineers had to negotiate. Making the process commercially viable has also depended on achieving several key objectives that range from placing strict limits on the size of the plant to choosing continuous rather than batch processing.
The company’s technology grew out of work originally done by engineers at the University of Warwick. Adrian Griffiths, Founder and Chief Executive of Recycling Technologies, saw its potential and raised the money to build a laboratory-scale unit, before developing its current beta plant in Swindon. The local borough council provided some of the first plastic waste to be treated by the plant at a rate of more than two tonnes per day.
Using pyrolysis to process plastic
The effectiveness of a chemical breakdown process often depends on finding the right catalyst to speed it. The Recycling Technologies machine uses no catalyst; it relies solely on heat. This eliminates the risk of catalytic poisoning in which unwanted compounds in the material being processed bond to the active surface sites of the catalyst, reducing its effectiveness in promoting the desired molecular breakdown. When, as here, the raw material is a waste product, you never know exactly what you might be getting – dyes, adhesives, fire-retardants and other potential catalytic poisons – in addition to the plastic itself.

The technology behind the RT7000 was developed by chemical engineers at the University of Warwick © Recycling Technologies
Another strategic decision in designing the RT7000 concerned the nature of the product to be extracted at the end of the process. One potential output is diesel fuel, and this is what some would-be chemical recyclers have opted for but have not managed on a commercial scale; when there is a varied input of plastic waste, it is extremely difficult to keep to diesel’s tight specifications.
Here, the company’s ‘less is more’ approach points to an alternative strategy: to produce a product with the least rigorous specification at which it can still find a buyer. The material that first emerges from the cracking process is too diverse to be easily marketable. By talking to potential buyers, Recycling Technologies found that separating the breakdown products of the waste plastic into four fractions would provide four different materials, with a market for each.
The plant recycles plastics of all kinds that have been separated from other waste, shredded, washed and dried. People might imagine that the first step of the process would be straightforward – feeding the waste into the vessel where the thermal cracking takes place – but pushing shredded plastic fragments into a container at 500ºC without simultaneously allowing air in turned out to be tricky. Any air in the vessel would turn the chemical process from pyrolysis to combustion.
pushing shredded plastic fragments into a container at 500ºC without simultaneously allowing air in turned out to be tricky. Any air in the vessel would turn the chemical process from pyrolysis to combustion
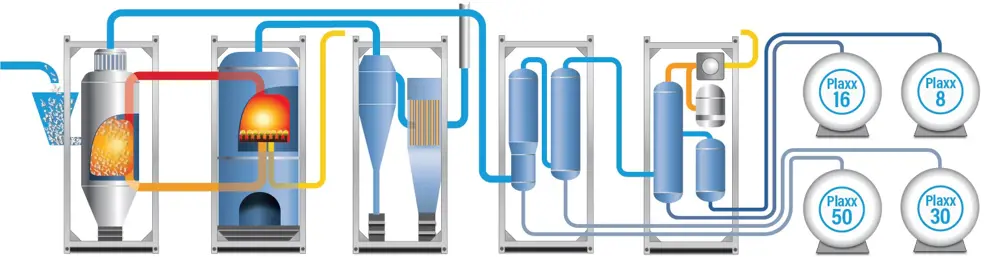
The pyrolysis process, also known as 'thermal cracking', uses heat in the absence of oxygen to break down plastics © Recycling Technologies
A screw-feed system that compresses the waste plastic and drives it into the heating vessel seemed to be the obvious way forward. However, conventional screw feeds do not work well with shredded fragments of mixed plastic in a range of shapes and sizes, and in physical forms ranging from film to particles. The RT7000 relies on a tapered screw to move the plastic mixture forward, compress it, and force out pockets of air. Under the high pressure, the plastic becomes warmer and softer, and can then be squeezed into the reaction chamber in the manner of a stiff toothpaste emerging from a nozzle. The company spent two years developing the system and will be patenting it when fully satisfied that all the wrinkles are ironed out.
Most previous attempts to pyrolyse plastic have used batch processing, in which set quantities of material are fed into the reaction chamber and treated load by load. This is time-consuming and more expensive than the continuous processing favoured by Recycling Technologies.
Fluidised bed technology, widely employed by industry for a variety of processes ranging from the gasification of coal to the manufacture of fertilisers, makes a mass of particles behave as if they were liquid
To heat the plastic inside the reactor vessel, and simultaneously create an even temperature throughout, a very hot and sand-like material is pumped into the vessel using a fluidised bed system. Fluidised bed technology, widely employed by industry for a variety of processes ranging from the gasification of coal to the manufacture of fertilisers, makes a mass of particles behave as if they were liquid. This is achieved by pumping a fluid – either a gas or a liquid – up through the container holding the particles. When the rate at which the fluid entering the container reaches a critical level the particles within it can be stirred or pumped. A football dropped on to bed of fluidised sand will create a splash, and then float on it.
Sand was the material originally chosen for use as the heating medium in the RT7000. In a container next to the main reaction vessel, the sand was both heated using propane gas and fluidised by forcing the gas through it. The hot fluidised sand was then pumped into the reactor, so mixing with and heating the particles of waste plastic and causing their molecules to fragment into a vapour of various hydrocarbons. Sand that had given up its heat was pumped out of the reactor vessel and back into the heating chamber. However, it soon became clear that sand was not the ideal material for these purposes, and the machine now uses specially synthesised ceramic particles the size of sand grains, but with a more uniform shape. Once the process is up and running at the required temperature, gases released during the thermal cracking process heat the ceramic ‘sand’, and the propane can be phased out as the process generates its own fuel supply.
One of the key variables in the system is the rate at which heat transfers between the carrier and the plastic. The hydrocarbon vapours released by the cracking of the molecules of plastic are piped to refining columns to remove unwanted compounds such as fire retardants and, most importantly, the chlorine from PVC. This hot-gas filtration system for removing chlorine and other halides is something that the company has had to devise to suit its particular needs. This requirement was vital because of the company’s claim to be able to deal with plastic waste of any kind, including (up to a point) PVC. Experience shows that domestic waste in general contains no more than 1% to 5% of PVC, which the RT7000 should be able to cope with. Levels much higher than this might require the waste to be pre-sorted to remove most PVC.
The final product
The final distillation stage of the process involves the separation of the hydrocarbon vapour into the four different materials. These are:
- A light oil suitable for use by the plastics industry as feedstock for new plastic production.
- A low-sulphur marine gas oil suitable as a fuel for marine engines or heat and energy generation.
- A low-sulphur heavy fuel oil.
- A hydrocarbon wax suitable for coatings and environmental protection, as a bitumen modifier, and even for candles.

The chemically recycled plastic is broken down into oil and wax fractions. The company has forward sold all the oil and wax product from its first 12 machines to customers in polymer and wax manufacturing for further refinement © Recycling Technologies
The distillation column splits into four separate units standing side by side. This arrangement means that no one column needs to be particularly tall: a helpful factor in seeking the planning permission required to set up a new plant. Of the original plastic, about 3% remains to be disposed of as a hazardous waste. A key point is that Recycling Technologies is not aiming to produce products to a very tight specification, which would increase the cost and complexity of the plant. Buyers of the product – who know what they are getting and will pay accordingly – are better placed to perform any further purification or refinement according to their needs.
Plastics by numbers
🪀 Different types, uses and properties of plastics
- Polyethylene terephthalate (PET) Clear, strong material. Barrier to moisture and resistant to heat and solvents. Used for water and soft drink bottles, pots, tubs etc. Commonly recycled.
- High-density polyethylene (HDPE) Strong, stiff material resistant to chemicals and moisture, but permeable to gases. Used for toys, household and kitchenware, carrier bags, food wrapping. Commonly recycled.
- Polyvinyl chloride (PVC) Tough material, resistant to grease, oil and chemicals. Rigid, but can be made flexible. Good transparency. Used for window frames, water and drainage pipes, flooring, roofing membranes, car interiors and seat coverings, footwear, packaging, and coated fabrics. Sometimes recycled.
- Low-density polyethylene (LDPE) Soft material, easily processed with good flexibility, easily sealed. Used for squeeze bottles, toys, carrier bags, heavy duty sacks, general packaging, gas and water pipes. Sometimes recycled.
- Polypropylene (PP) Versatile material, barrier to moisture, resistant to chemicals. Plastic that it easily moulded for a wide variety of uses. Can be recycled.
- Polystyrene (PS) Hard and brittle material with good clarity and easily foamed. Widely used as packaging and insulation material. Can be recycled, but with difficulty.
- The rest All other types of plastic. There may be as many as 1,000, including some materials manufactured for exotic purposes.
Plant manufacture and commercial viability
The Swindon beta plant is the prototype, using the suppliers, equipment and standards, for the full-scale commercial RT7000 plant. Recycling Technologies does not envisage a situation where plastic is trucked in from a large area to a central recycling centre. Instead, the commercial plant is about the size that would be required by most borough councils, and installation at an existing recycling site would locate it where the plastic is first separated from the rest of the rubbish. This positioning should also minimise planning permission problems that might be encountered if seeking to install the equipment on a virgin site.
The potential annual energy saving that could be achieved from recycling all global plastic waste is equivalent to 3.5 billion barrels of oil worth approximately $176 billion

Keeping plastics in the circular economy: chemical recycling enables waste plastics to be converted to oil for making virgin-quality polymers, suitable for all applications, including food-grade plastics © Recycling Technologies
The company aims to manufacture plants on an assembly line basis. Customers would buy what amounts to an off-the-shelf unit, typically for use on an existing recycling site. As this is a field in which innovation is still occurring, future units would incorporate improvements as they became available. The precise economics are still speculative, but if machines were to cost £3 million to install and £500,000 annually to operate, each might produce a revenue of £1.7 million every year from product sales. The plant should pay for itself in less than three years: a speedy recovery of the initial capital. According to present plans, the first RT7000 will be installed in Perthshire and be designed to handle 7,000 tonnes of plastic waste annually.
In summary, making the process commercially viable has depended on several key goals: keeping the plant simple; keeping it small enough for mass manufacturing, so reducing the capital cost; distributing units geographically rather than centralising them; and running them continuously.
A recent Nature review of plastics recycling from the University of Houston’s Department of Chemical and Biomolecular Engineering drew attention to the advantages of chemical recycling methods. They have lower energy requirements and can handle mixed plastic waste, its authors wrote, and they can encompass traditionally non-recyclable polymers. “The potential annual energy saving that could be achieved from recycling all global plastic waste is equivalent to 3.5 billion barrels of oil worth approximately $176 billion.”
While it may be overstating things to claim that “there’s gold in them thar plastics”, environmental dumping of waste plastic is not only a criminal activity but also, it would seem, a neglected opportunity.
***
This article has been adapted from "Processing the plastic problem", which originally appeared in the print edition of Ingenia 75 (June 2018).
Contributors
Geoff Watts
Author
Adrian (Rupert) Haworth is a Marketing and Strategy Consultant at Gazelle Wind Power Limited and the Founder of Stratergy Ltd. At the time of writing he was Sales and Marketing Director at Recycling Technologies. He spent 33 years with GE, covering various roles including Engineering Services Manager, Director of European Service Sales, Region Director for Russia/CIS and Director of GE Energy’s Strategic Marketing department in Europe.
Keep up-to-date with Ingenia for free
SubscribeRelated content
Environment & sustainability

Recycling household waste
The percentage of waste recycled in the UK has risen rapidly over the past 20 years, thanks to breakthroughs in the way waste is processed. Find out about what happens to household waste and recent technological developments in the UK.

Upgrade existing buildings to reduce emissions
Much of the UK’s existing buildings predate modern energy standards. Patrick Bellew of Atelier Ten, a company that pioneered environmental innovations, suggests that a National Infrastructure Project is needed to tackle waste and inefficiency.

An appetite for oil
The Gobbler boat’s compact and lightweight dimensions coupled with complex oil-skimming technology provide a safer and more effective way of containing and cleaning up oil spills, both in harbour and at sea.

Future-proofing the next generation of wind turbine blades
Before deploying new equipment in an offshore environment, testing is vital and can reduce the time and cost of manufacturing longer blades. Replicating the harsh conditions within the confines of a test hall requires access to specialist, purpose-built facilities.
Other content from Ingenia
Quick read

- Environment & sustainability
- Opinion
A young engineer’s perspective on the good, the bad and the ugly of COP27

- Environment & sustainability
- Issue 95
How do we pay for net zero technologies?
Quick read

- Transport
- Mechanical
- How I got here
Electrifying trains and STEMAZING outreach

- Civil & structural
- Environment & sustainability
- Issue 95