
How does steelmaking work?
Traditionally, steel is made in a blast furnace, but electric arc furnaces are increasingly used as they create less carbon emissions. Steel is an alloy of mostly iron and carbon. Pure iron is soft and weak: the carbon is essential for its strength. However, a carbon content higher than about 2% makes it brittle. So, steelmakers mix up the proportions according to the client’s needs. Adding other elements such as manganese, chromium and nickel can improve its properties, making it harder or more corrosion resistant.
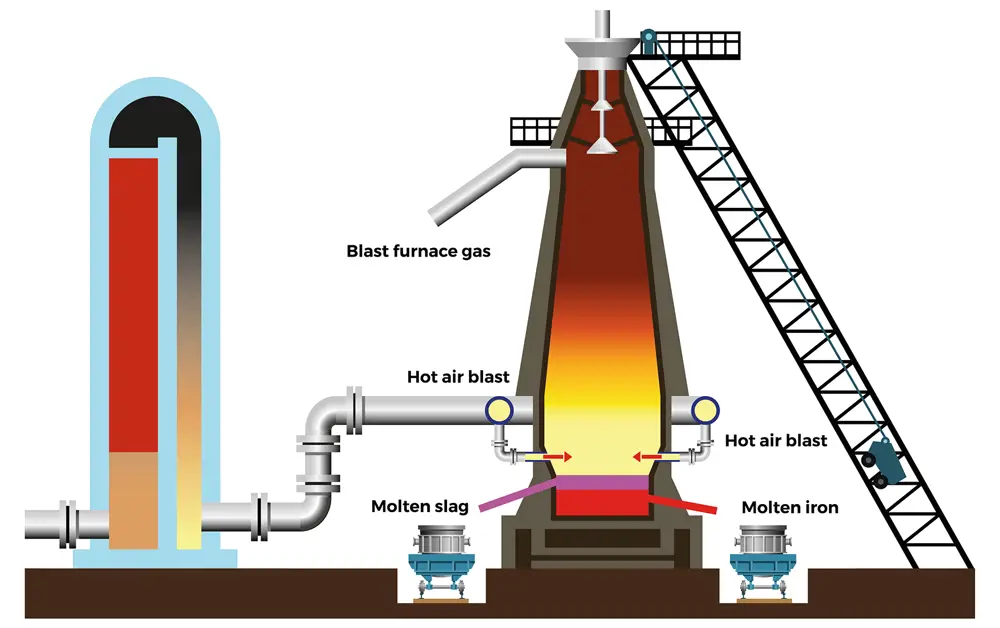
Simplified schematic of a blast furnace. A hot stove (left hand side) is feeding a hot air blast into a blast furnace (right hand side), which produces molten slag on the left and molten iron on the right © Shutterstock
There are two main steps to blast furnace steel production. The first is extracting iron from iron ore mined from the earth. A mixture of iron ore, coke (a type of coal that has been baked to drive off impurities such as tar), and limestone are fed into the top of the blast furnace in a specific order. This is called ‘charging’ the furnace. At the same time, a stream of air heated to about 1,200°C by a device called a hot stove (the ‘blast’) is supplied to the bottom of the furnace.
As the column of coke, iron ore and limestone descends towards the blast, the coke ignites. The burning fuel in the furnace raises the temperature to about 2,200°C and produces carbon monoxide. Oxygen from the iron ore reacts with the gas to produce carbon dioxide, leaving molten iron, which flows out of the bottom of the blast furnace. Impurities from the ore react with the limestone and coke ash to form slag, which floats on top and can be removed. Because of an excess of carbon, some of the CO2 is reduced back to carbon monoxide and emitted from the top of the furnace.

Molten iron and slag flow out of the bottom of the blast furnace into a vessel called a 'ladle' © Shutterstock
The coke also plays a structural role within the column of materials added to the furnace: ensuring the blast passes evenly through the iron ore. To reduce costs and lessen the coke needed, pulverised coal is often injected into the blast furnace with the hot blast. At this stage, the carbon content of the molten iron is at about 4%, so must be decreased.
Next, the hot metal is treated to remove impurities such as sulphur. It is then added to a vessel containing some scrap steel (known as a basic oxygen furnace) – whose role is to lower the temperature, while boosting the iron content. Then, a water-cooled pipe lowered into the vessel blows oxygen onto the liquid metal at about twice the speed of sound. This oxidises the carbon in the hot metal, forming carbon monoxide and CO2. After 20 minutes or so, steelworkers check whether the liquid steel has reached the desired chemical composition. It may then be refined further, for example, by adding alloying agents.

Steel is rolled at a high temperature © Shutterstock
Liquid steel is then cast into slabs and can be cut and rolled to form different shapes depending on its ultimate use. Large panels may be used for cars, thin sheets to form tin cans, or bars and beams for construction.
In the overall process, the blast furnace accounts for over 70% of CO2 emissions due to the chemical reduction process and the fuel required to heat the furnace. The basic oxygen furnace is less energy intensive. Typically, it does not consume extra energy, as oxidising the carbon in the molten iron creates heat.
Contributors
Leonie Mercedes is a freelance writer based in London.
Keep up-to-date with Ingenia for free
SubscribeRelated content
Design & manufacturing

Super cool(er)
Welsh startup Sure Chill has developed a cooler that uses the properties of water to keep its contents cool for around 10 days without electricity. This is ideal for storing items such as vaccines where electricity sources are unreliable.

R&D investment makes good business sense
In just five years, Dr Ralf Speth FREng has presided over a revolution in design and manufacturing that has helped create a new family of engines and has overhauled Jaguar Land Rover (JLR) production facilities.

Steel can arise from the ashes of coal
Thousands of people were laid off in the UK steel industry in 2015 and there are pessimistic future forecasts. Professor Sridhar Seetharaman of the Warwick Manufacturing Group argues that smaller, flexible steel mills implementing new technology would better cope with fluctuating global trends.

Integrating metrology in business and academe
Professor Jane Jiang’s interest in measuring began when she worked on a bus production line in China. She found that the best way to improve quality, consistency and productivity was through metrology, the science of measurement. Today, she runs the UK’s largest metrology research group.
Other content from Ingenia
Quick read

- Environment & sustainability
- Opinion
A young engineer’s perspective on the good, the bad and the ugly of COP27

- Environment & sustainability
- Issue 95
How do we pay for net zero technologies?
Quick read

- Transport
- Mechanical
- How I got here
Electrifying trains and STEMAZING outreach

- Civil & structural
- Environment & sustainability
- Issue 95