
An easier way to diagnose disease
The underlying idea behind Owlstone Medical’s innovative breakthrough in medical diagnostics is, in the words of Billy Boyle, its CEO and co-founder, that “every chemical has its own smell ‘fingerprint’”. At first, Boyle and his colleagues at the University of Cambridge established Owlstone Inc to create chemical sensors for military systems. Medical diagnostics came later. In 2004, the researchers set up the company to commercialise their academic work in micro- and nano-engineering, developing small and inexpensive sensors on silicon chips. Their ambition was to swap expensive sensors and systems in devices that cost around £10,000 for sensors that cost a few pounds.
Their ambition was to swap expensive sensors and systems in devices that cost around £10,000 for sensors that cost a few pounds
Owlstone’s aim was always to protect the public. The idea was that it could play a role “protecting every train, financial institution, government building, airport, stadium and indeed any target at risk from chemical or explosive attack”. Owlstone’s sensors could detect some 20 different chemical ‘fingerprints’, which meant that it could check simultaneously for sarin and mustard gas, for example.
Owlstone Inc went on to build a successful, and now profitable, business in chemical sensing for customers in defence, security and business. Over the past decade, the company has raised $38 million in investment and won defence contracts worth more than $32 million. The engineering promise of that early progress was such that Owlstone was one of the three engineering innovations shortlisted for the MacRobert Award in 2008.
A family tragedy accelerated Owlstone’s move into medical diagnostics. Boyle’s wife died of colon cancer because of a late diagnosis, and Boyle admits that anger spurred him on after his wife’s death. “Anger is an energy: it is what drives me and my team,” he confesses. He started working with medical experts to devise ways of diagnosing cancers earlier as: “early detection is our greatest opportunity”. Another important benefit of Breath Biopsy is its patient-friendly nature. “Breath is the ultimate non-invasive test,” says Boyle.
“early detection is our greatest opportunity”. Another important benefit of Breath Biopsy is its patient-friendly nature. “Breath is the ultimate non-invasive test,” says Boyle
From the outset, Owlstone’s ‘manifesto’ had included medical applications, but that was a far more challenging goal, not least because despite a long history as a possible diagnostic technique, the medical world had yet to accept the idea of ‘breath diagnosis’. Boyle decided to do something about it. The argument is straightforward: metabolic processes in the body create a very large array of different chemicals depending on what is going on. These complex chemicals enter the bloodstream then flow around the body, hence entering the lungs, which act as an oxygen exchanger for the blood. “Your lungs are very good at exchanging gas from blood to the airways,” says Boyle. “Every time you breathe out there are thousands of volatile organic compounds (VOCs) in your breath.” Underlying changes in metabolic activity can produce patterns of VOCs that are characteristic of specific diseases.
While Owlstone’s founders always thought that their sensors might play a role in medical diagnosis, they had to work out how to do it. Perhaps even more importantly, they also had to prove not just that their approach made medical sense but persuade the medical community that it would work. In essence, Owlstone had to devise a new form of medical diagnostics based on chemical analysis of the VOCs in breath.
Breath breakthroughs
Over the years, there have been many newspaper reports of ‘breaththroughs’ in the development of an electronic nose, but they rarely go further than the headlines. One review article summed up the situation not long after Owlstone opened for business: “Despite the success in some areas, the efforts to arrive at a universal device that can make fine discrimination of flavours, perfumes, and smells and eventually replace the human nose are disappointing.”
By the time Owlstone turned its attentions to medicine, it had overcome some of that disappointment. But medical diagnostics is a very different market to sampling air to find harmful chemicals. Medical systems require long and expensive trials to take new technology through the regulatory processes that are essential for diagnostic systems.
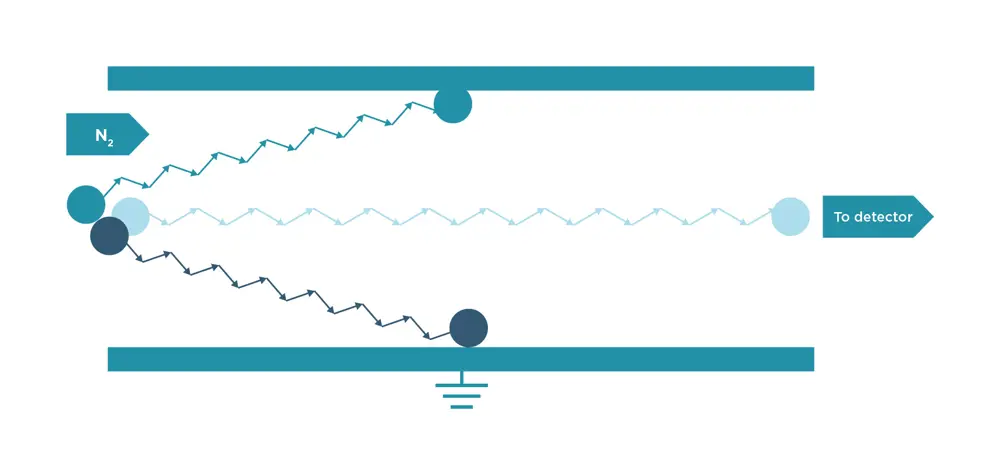
Figure illustrating how field asymmetric ion mobility spectrometry (FAIMS) works. The diagram shows three ions, all demonstrating different mobility behaviours under the influence of the electric field. The paths of two ions collide with walls, but the third ion has the appropriate DC voltage applied, meaning it traverses the device that scans these DC voltages, producing a compensation field spectrum for the transmission of all ions © Owlstone Medical
It was only in 2016, more than a decade after it opened for business, that Owlstone’s founders set up a new medically oriented company, Owlstone Medical. The idea that you could use breath sampling to diagnose diseases has been around for some time – Hippocrates (460 to 370 BC) first linked breath odour to underlying diseases – but it got nowhere: as well as small and inconsistent studies, the most significant issue was a lack of a standardised technology for collecting high-quality breath samples and analysing them reproducibly. Before Owlstone could tackle that challenge, it had to adapt its existing sensors and create an optimised device that could take a sample containing VOCs and give a reliable analysis of what was in a breath sample.
The platform includes a patented chemical sensor on a silicon chip, based on a technique called field asymmetric ion mobility spectrometry (FAIMS). Owlstone’s technology revolutionised ion mobility spectrometry by turning to micro- and nano-fabrication to miniaturise the spectrometer and its channels, while integrating tiny detectors into the device along with a solid-state FAIMS filter. The result is a programmable sensor on a silicon chip. Tweaking the software can program the chip to instantly detect different chemicals. FAIMS is a variant of ion mobility spectrometry, where a tiny radioactive source ionises the VOCs in a breath sample. By applying an alternating electric field across the path of the ions, the chip can act as a filter that allows only certain ions through to the sensor.
The ionised VOC gas passes through channels under the influence of an asymmetric radio frequency field. Under the first portion of the waveform, ions will drift in one direction at a speed based on their individual mobility in that electric field. As the field polarity is reversed, the ions change direction and speed based on the new field conditions. As the mobility of the ions during the two parts of the waveform is rarely equal, there is usually a net drift towards one of the electrodes. In FAIMS, this net drift is corrected by applying an additional DC voltage, known as the compensation field, focusing specific ions through the device to the detector. The device scans the DC voltages to produce a spectrum that reveals the VOCs that are present in the sample.
Taking a sample
The sensors and measurement system on their own are of little value without a reliable and reproducible way of capturing the VOCs in breath. Owlstone developed its ReCIVA® Breath Sampler device, one of the innovations for which it won the MacRobert Award, to fill this role and to create a standardised breath sampling and analysis procedure. Subjects breathe a controlled supply of air, and samples of their exhaled breath are captured on Breath Biopsy cartridges, which can then be analysed to determine their VOC profile.
When developing the breath sampler, Owlstone had to pay careful attention to the cost of the cartridge that captures the VOCs, as disposable cartridges would have been too expensive. The solution was to devise cartridges that can be reconditioned and sent out to be reused.

The ReCIVA Breath Sampler monitors patient breathing and automatically selects a fraction of expired breath to be captured. Breath is directed into four collection tubes, which are packed with different sorbents that are optimised to collect volatile organic compounds in breath © Owlstone Medical
The design for the ReCIVA Breath Sampler drew on discussions with more than 100 multidisciplinary experts in breath diagnostics. The result is a breath sampler that is becoming the industry standard for the reliable and consistent collection of breath samples. The standardised device for breath sample collection was essential if breath analysis was to be effectively deployed in clinical trials. As Owlstone describes it: “this also means that we now have technology that is robust enough to collect reliable breath samples in clinic, and identify biomarkers for disease in breath”. A key feature is the reduction in the variability of the measurements, allowing statistically predictable decisions to be made about response to treatment.
The device consists of a base unit that can be attached to a PC or tablet to control and monitor the sampler. The ReCIVA also has a positive pressure air compressor for patients who need it as well as a disposable mask with a built-in bacterial filter to prevent contamination of the base unit and a cartridge for collecting the sample. Owlstone’s goal is to measure traces of VOCs in a quantifiable manner from precisely sampled breaths. A critical feature is the ability to reduce sources of potential contamination in the whole collection cycle.
The ReCIVA base unit monitors the patient’s breathing by checking the pressure and by measuring CO2. It automatically selects which section of the expired breath to capture. It then controls piezoelectric valves to direct the breath sample into one of the four collection tubes that form the Breath Biopsy cartridge.

Owlstone hosts ‘the world’s largest Breath Biopsy digital biobank’, which is a valuable resource available to researchers using its Breath Biopsy Services © Owlstone Medical
Shared expertise
The technology was just the beginning of Owlstone’s challenge. It also had to persuade medics that Breath Biopsy made sense, which meant working with doctors to understand which VOCs could act as biomarkers for specific diseases. The company did not have to start from square one when it set out to make the case for its Breath Biopsy platform. There is a sizeable body of literature on the medical use of VOCs, including a dedicated Journal of Breath Research. Plenty of papers describe research into the use of VOCs, some of them based on Owlstone’s technology, but the concept had never made it into regular medical practice.
Owlstone is helping to change that. The current research activity in the area is one indicator that medical researchers take the idea seriously. As well as clinical trials, over 100 clinical sites across the world use Owlstone Medical’s Breath Biopsy technology. Pharmaceutical companies, clinical research departments in hospitals, academic researchers and other commercial organisations have taken up the idea for their own research in clinical trials and precision medicine applications.
One example of the take up of the diagnostic platform is GSK, which recently chose to integrate it into the clinical development programme of one of the novel drug candidates in its respiratory disease pipeline, to assess whether the right treatment for the right patient can be identified. AstraZeneca is also using Breath Biopsy to identify novel biomarkers for personalised medicine applications in asthma and chronic obstructive pulmonary disease. Another sign of the company’s efforts to spread the word was the recent Breath Biopsy Conference 2018, which it described as ‘Owlstone Medical’s inaugural community meeting for breath researchers’.
The final piece of the jigsaw was to develop a way to turn Owlstone’s engineering into a profitable model. The company does not just sell breath samplers and FAIMS instruments. In early 2017, Owlstone Medical launched its Breath Biopsy Services, so that academics, clinicians and pharmaceutical companies can explore breath-based diagnostics.
Researchers who want to implement Breath Biopsy have various options. They can lease the ReCIVA Breath Sampler and buy biopsy kits to collect breath samples. When it comes to analysing samples, users can either send them to Owlstone’s labs or they can conduct onsite analysis with the Lonestar® VOC Analyzer.
With $400 billion a year wasted on ineffective drugs, there is a global move towards precision medicine – ensuring that the right intervention and drug are given to the right patient at the right time
Owlstone in numbers
💸 Funding and lifesaving statistics
- More than 100 breath diagnostics experts support development of the ReCIVA Breath Sampler.
- $73 million in total funding raised since 2016.
- Up to 4,000 patients in NHS-supported LuCID lung cancer trial.
- ReCIVA units deployed in more than 100 sites.
- The Breath Biopsy platform aims to save 100,000 lives and $1.5 billion in healthcare costs.
Global growth
With so much going on in its Breath Biopsy platform, Owlstone has naturally worked hard to protect its intellectual property rights with, at the last count, nearly 40 patents. The company has set itself ambitious targets. It aims to save 100,000 lives and $1.5 billion in healthcare costs.
Development on this scale takes money, not least because it involves taking a whole system approach that takes in health economics as well as the development of the technology and supporting services. With a team of just over 150 people in Cambridge and plans to set up operations in the US and other countries, the company has been seeking investors. Not long after Owlstone Medical collected the MacRobert Award, it finalised another round of fundraising, with $50 million raised. The company has raised around $73 million since its creation in 2016.
The funding round also attracted investors from promising markets for Breath Biopsy, including China. Boyle said: “We enjoyed particularly strong interest from Asian investors in the round, and as China is a key market for the company, we are encouraged by the strategic value of these partners as we seek to accelerate entry into this market.” The new funding will also support the creation of a new analytical laboratory in the US.
Professor Sir Saeed Zahedi certainly sees plenty of promise in this new approach to medical diagnosis. “The future road map of this innovation in medical device technology is transformative and could be the key for future self-diagnostics as part of a long-life care revolution.”
One outcome of Owlstone’s analysis service has been ‘the world’s largest Breath Biopsy digital biobank’ that relates VOCs on breath to particular diseases. This resource is available to researchers who send samples for analysis and want to explore biomarker classifiers and gain valuable insights into how they might perform in larger patient populations. The biobank includes thousands of breath VOC profiles matched to phenotype. As Owlstone collects and analyses all VOCs on breath, each sample becomes a snapshot of the health of an individual at a moment in time. In this way, it enables studies performed by Owlstone, or on behalf of clients, to be augmented with in silico patient breath samples, and offers the potential to test hypotheses in the biobank without the need for additional patient samples. It is helping to overcome some of the historical challenges associated with identifying VOCs and relating how these are linked with specific diseases.
The benefit of the digital biobank and Breath Biopsy can go beyond disease diagnosis. There is growing interest in the idea that genetics can affect patients’ responses to different therapies. Owlstone believes that Breath Biopsy could help to identify patients most likely to respond to a particular therapy. Not only would this deliver better outcomes for patients, it could also reduce the costs of treatment, for example by reserving the use of expensive drugs for patients who are more likely to respond. Professor Sir Saeed Zahedi OBE RDI FREng, one of the MacRobert Award judges and Technical Director of Blatchford, explained: “With $400 billion a year wasted on ineffective drugs, there is a global move towards precision medicine – ensuring that the right intervention and drug are given to the right patient at the right time.”
***
This article has been adapted from "An easier way to diagnose disease", which originally appeared in the print edition of Ingenia 77 (December 2018).
Contributors
Michael Kenward OBE
Author
Billy Boyle is an engineering graduate from the University of Cambridge. He is one of the original co-founders of Owlstone Inc, spun out of Cambridge in 2004. In 2016, Billy led the process to spin-out Owlstone Medical Ltd and became the founding CEO.
Keep up-to-date with Ingenia for free
SubscribeRelated content
Health & medical

Kidney dialysis
Small haemodialysis machines have been developed that will allow more people to treat themselves at home. The SC+ system that has been developed is lighter, smaller and easier to use than existing machines.

Engineering polymath wins major award
The 2015 Queen Elizabeth Prize for Engineering has been awarded to the ground-breaking chemical engineer Dr Robert Langer FREng for his revolutionary advances and leadership in engineering at the interface between chemistry and medicine.
Blast mitigation and injury treatment
The Royal British Legion Centre for Blast Injury Studies is a world-renowned research facility based at Imperial College London. Its director, Professor Anthony Bull FREng, explains how a multidisciplinary team is helping protect, treat and rehabilitate people who are exposed to explosive forces.
Targeting cancers with magnetism
Cambridge-based Endomag has helped treat more than 6,000 breast cancer patients across 20 countries. The MacRobert finalist uses magnetic fields to power diagnostic and therapeutic devices. Find about the challenges that surround the development and acceptance of medical innovations.
Other content from Ingenia
Quick read

- Environment & sustainability
- Opinion
A young engineer’s perspective on the good, the bad and the ugly of COP27

- Environment & sustainability
- Issue 95
How do we pay for net zero technologies?
Quick read

- Transport
- Mechanical
- How I got here
Electrifying trains and STEMAZING outreach

- Civil & structural
- Environment & sustainability
- Issue 95