Targeting cancers with magnetism
The medical technology company Endomag uses magnetic fields and tiny magnetic particles to evaluate the progression of breast cancer. By developing a magnetic tracer and a detector that can find the tracer in the body, it has created technology that simplifies cancer diagnosis. The first products commercialised from this platform – the Sienna+ tracer and Sentimag detector – make it easier to confirm whether cancer has spread. The company’s first target for its technology was to improve the diagnosis of breast cancer. The medical community is now adopting Sienna+ and Sentimag for other cancers.
The medical technology company Endomag uses magnetic fields and tiny magnetic particles to evaluate the progression of breast cancer
Sentimag probe
🧲 The engineering challenges of the Sentimag probe
The Sentimag operates by radiating an alternating magnetic field from drive coils in the probe. The oscillating field energises the nanoparticles trapped in the sentinel lymph nodes. A separate sense coil picks up a response that correlates to the particles’ magnetic susceptibility.
There are many challenges in engineering the co-axial drive and sense coils. One is to isolate the two sets of coils. The drive coils put out a signal that is a million times greater than the signal that the sense coil is looking for. Another complication is that there are also stray magnetic fields from other electronic equipment in the operating theatre. Still another signal comes from the subtle magnetic fields that water in body tissue produces in response to the drive field.
Careful signal processing overcame some of these challenges, but the probe is also sensitive to submicron movements in the coils. These tiny movements can come about as a result of temperature changes when the probe is in contact with the patient, for example. To reduce these effects, Endomag has developed different approaches to material selection for its probes. The latest version uses a cylindrical ceramic core with high thermal conductivity so that the temperature quickly stabilises in use, but so that the core remains stiff enough to machine and maintain an exacting coil geometry.

The Sentimag consists of a probe connected to a signal processing unit. The probe is a cylinder 18 mm in diameter and 100 mm long, which is sheathed during surgery. The signal processing unit has an LCD display that indicates the detected signal with a corresponding audible tone that rises in pitch with the signal intensity. There is also a button to ‘balance’ the system to set the detected signal to zero © Endomagnetics Ltd
Magnetic nanoparticles
Endomag has had to overcome many challenges since 2007, when it span out of research being conducted at University College London and the University of Houston, Texas. One of its first tasks was to develop an instrument that could detect less than a tenth of a milligram of magnetic nanoparticles 3 cm deep in body tissue. Each nanoparticle has a diameter of around 60 nanometres, about one hundredth the size of a red blood cell. The nanoparticles consist of a small 5–10 nm core of superparamagnetic iron oxide, coated with sugar to make the nanoparticles biologically compatible. The particles have similar dimensions to viruses and are naturally filtered out, like foreign invaders, in the lymph nodes.
The original prototype magnetic detector, devised by Simon Hattersley at University College London and Audrius Brazdeikis at the University of Houston, relied on a superconducting quantum interference device sensor that requires cryogenic cooling to operate. Given the requirement for a flask of liquid nitrogen, the early prototypes were not practical or safe for use in an operating theatre. In 2009, Hattersley devised a way of using a room-temperature sensor to achieve similar sensitivity by using exacting tolerances in the probe’s induction coils. This required a novel sensor design alongside a specialised signal processing system and, by 2010, the team had developed the Sentimag – see Sentimag probe.
The Sentimag is the world’s most sensitive handheld sensor of magnetic susceptibility – a measure of the degree to which a material responds to a magnetic field. The challenge in developing Sentimag was to come up with a detector that could reliably distinguish the minute magnetic signature produced by the magnetic particles in the body as they responded to the magnetic fields radiated by the device.
The Sentimag is the world’s most sensitive handheld sensor of magnetic susceptibility – a measure of the degree to which a material responds to a magnetic field
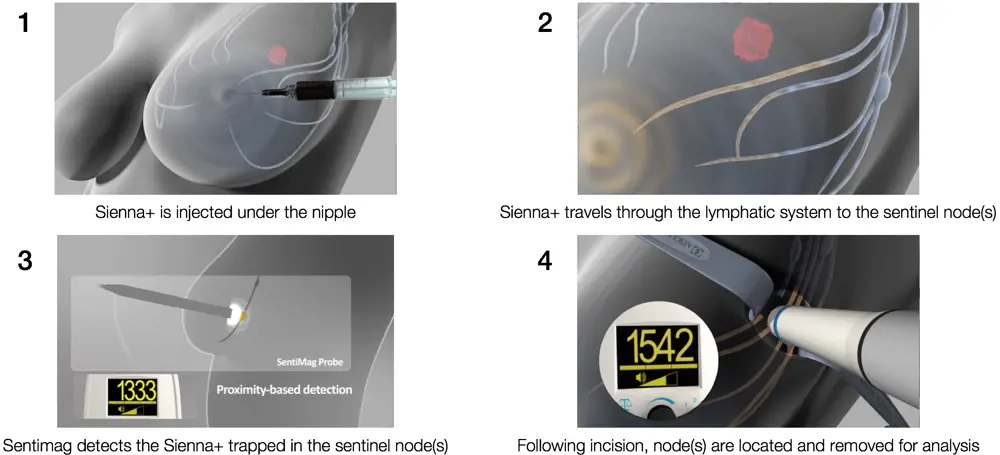
Endomag’s diagnostic system for breast cancer relies on an injectable fluid, branded as Sienna+, that contains superparamagnetic iron oxide nanoparticles. These particles accumulate in the lymph nodes that are the first to receive drainage from a breast tumour – the ‘sentinel’ lymph nodes. The Sentimag then locates these nodes for removal so that clinicians can check for signs of the cancer spreading.
An important advantage of Sienna+ is that, unlike the conventional radioactive tracers used for detecting lymph nodes, it does not require nuclear medicine to administer the injection, or careful timing to match the half-life of the radioisotope to the surgeon’s availability. Moreover, Sienna+ has a three-year shelf-life, rather than the six-hour half-life of a radioactive tracer, so hospitals and clinics far from nuclear medicine facilities can carry out sentinel lymph node biopsy. After about 20 minutes from injection, the particles migrate to the sentinel lymph nodes. The surgeon then uses Sentimag, probing as one would use a metal detector, to locate and remove the sentinel lymph nodes that contain Sienna+.
Equipment for use in operating theatres has to be easy to use and robust. Endomag has made steady progress in the design of its electronic systems, leading to ever smaller equipment. The electronics engineering combines a specialised low-noise amplifier with adaptive signal processing in a design that evolved to match the changing specifications of the probe.
Approval process
This technology has pushed back barriers in many fields. It has raised questions such as; is the company a medical diagnostics business? Does it manufacture surgical devices? Could it even find therapeutic applications for its technologies? The answer is that the company operates in all three areas. This can make it hard for investors, the media and, sometimes, competition judges, to know how to categorise it. The company’s technology crosses boundaries in biology, chemistry and physics and several disciplines of engineering, materials science and electronics.
Like all new medical technologies and treatments, the company has to navigate an approvals process that is complicated by the different regulatory approaches in different countries. Endomag had the advantage of being regulated as a device rather than as a pharmaceutical. This significantly reduces the cost of obtaining regulatory approval. In 2011, Endomag achieved its first important regulatory landmark when it received approval for using nanoparticles as a ‘medical device’. It was the first company to receive consent for nanoparticles and to commercialise them widely in Europe. The company hopes to follow its European success on this front by becoming the first nanoparticle device to receive regulatory approval by the US Food and Drug Administration.
The company’s technology crosses boundaries in biology, chemistry and physics and several disciplines of engineering, materials science and electronics
There used to be a common understanding of what constituted a drug, with its chemical effects on the body, and what a device, with its physical intervention. That dividing line is starting to blur. At the nanometre scale, it is hard to differentiate between physical and chemical interactions. As a result, regulators have to write new legislation to approve the medical uses of nanomaterials.
The Sienna+/Sentimag system has also received an Investigational Device Exemption (IDE) from the US Food and Drug Administration. This is the first stage in the regulatory process in the US and allowed the company to start a pivotal clinical trial in support of full regulatory approval. The trial, underway at six sites across the US, is expected to conclude by the end of 2015. Endomag anticipates that it will receive regulatory approval in 2017, opening the way for a US commercial launch.
Sentimark
🍚 Marking tumours with a magnetic seed
Typically mammography and ultrasound are used to locate small tumours before surgery. The radiologist will mark the tumour for the surgeon by inserting a guide wire. These wires are uncomfortable for the patient and restrictive for the surgeon. The wires must be placed on the morning of the surgery, often causing OR delays. Also they can become dislodged as the patient is moved between departments, making the surgeon’s task more difficult and causing additional patient anxiety. Sentimark marks tumours with a magnetic seed about the size of a grain of rice. A radiologist puts the seed into the tumour up to 30 days in advance of surgery, guided by mammography or ultrasound. Thanks to the design of the seed, Sentimag can locate where it was placed and help the surgeon locate the tumour.
It took smart materials engineering to create a Sentimark seed that Sentimag can detect and that is also visible to X-rays and ultrasound. The seed was also designed so that tissue would integrate into the tumour’s structure to minimise movement. That structure handily increases the ultrasound signature. Sentimark was unveiled in September 2015, and it is currently undergoing its final stages of product approval in Europe and the US.
Business development
When it came to raising funds, despite this complicated picture, the company did find investors who understood its plans and were willing to invest over £7 million, so far, into the business. Fortunately, unlike many healthcare start-ups, Endomag does not have to rely solely on investors to fund its development as it already generates income from sales of Sienna+ and Sentimag. Since its first sales in 2012, the company has seen triple-digit growth. Selling to hospitals and clinics, it has already treated 10,000 breast cancer patients across Europe, the Middle East and Australia. In 2014, the company’s revenues exceeded £2.5 million.
As surgeons want to remove as little tissue as possible, they need to pinpoint tumours as accurately as possible
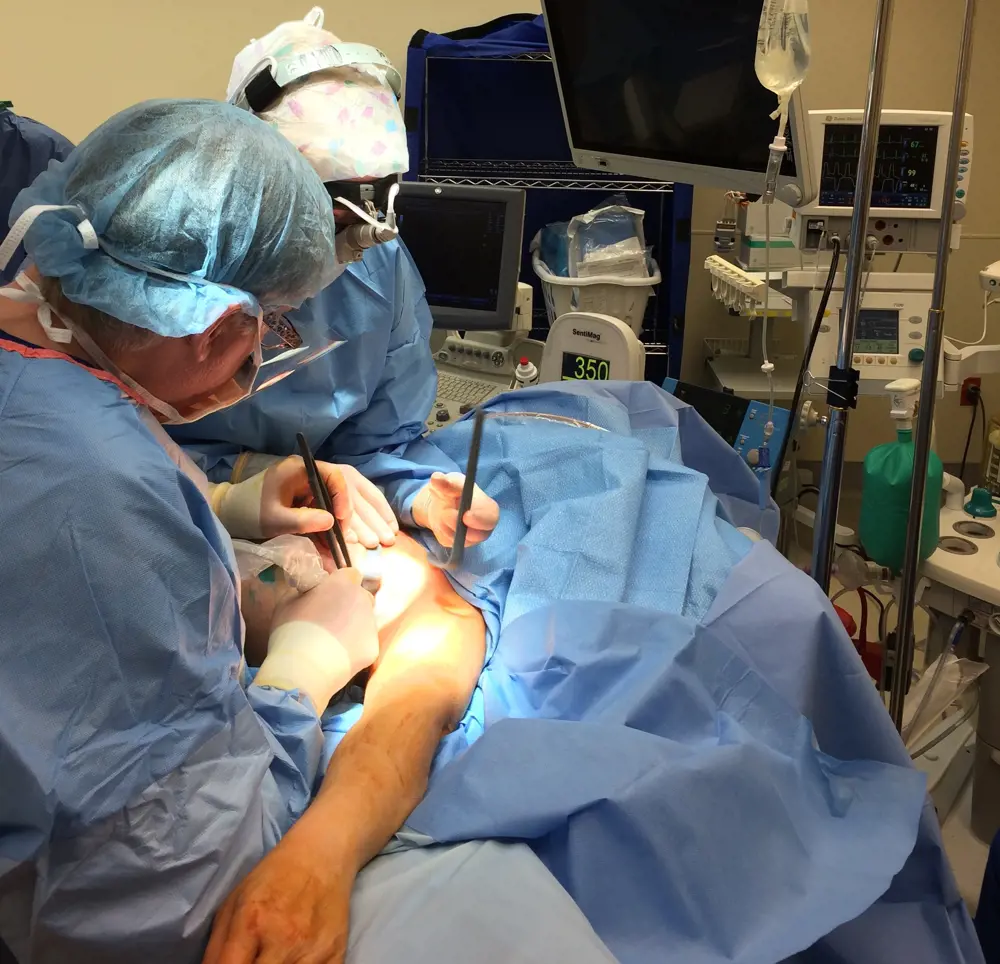
Dr Peter Beitsch uses the Sentimag to locate sentinel nodes in a breast cancer patient © Dr Peter Beitsch, Dallas Surgical Group
Endomag is now applying its underlying technologies for other medical applications. Next year, it plans to launch Sentimark, another innovation in the treatment of breast cancer. This new product came about partly because surgeons were asking the company for help to locate tumours as well as lymph nodes. Advances in screening for breast cancer now detect tumours earlier, when they are just a centimetre or two across, too small for surgeons to simply feel them. As surgeons want to remove as little tissue as possible, they need to pinpoint tumours as accurately as possible – see Sentimark.
Endomag has managed to develop its technologies and to negotiate the regulation processes with a relatively small staff. It achieves this by outsourcing some of its activities, including marketing and manufacturing the Sienna+/ Sentimag system. Now, the company is determined to grow through product development, manufacturing and sales rather than through licensing its intellectual property.
It is now developing its underlying technology platform in ways that will allow it to treat as well as locate cancers. Nanoparticles are similar in scale to the mechanisms of cancer, so Endomag is looking to build on established approaches, and existing pharmaceuticals, to detect and treat tumours. For example, magnetic nanoparticles can heat cancer cells in a way that does not kill them or the healthy surrounding tissue, but that makes the tumour more susceptible to chemotherapy. Interrupting the tumour’s ‘machinery’ makes the drug more effective and could mean delivering lower doses of traditionally toxic chemicals.
The treatment of other cancers also requires better ways of confirming the potential spread of tumours. Endomag has general approval for sentinel lymph node biopsy, which implies that doctors and surgeons can use the technology for any cancer. However, the company itself has taken a conservative strategy and has restricted its marketing to breast cancer, where it has evidence to show that its approach works.
This has not stopped surgeons from using the technology to investigate other cancers. While this means that they have to seek ethical approval before they do anything, surgeons have already started to use the Sienna+/Sentimag system in the treatment of prostate cancer. As clinical evidence becomes available from these activities by inquisitive surgeons, so long as it obtains approval, Endomag will be in a position to market its products for new conditions.
Thanks to this ‘viral uptake’ of Endomag’s medical devices, the company is developing advanced laparoscopic and endoscopic probes for other types of cancer including melanoma, prostate, thyroid, colon and cervical cancer.
***
🧲 Find out more - Endomag's website.
🩺 Update - Since this article was published, Endomag has been adopted by 1000+ hospitals, used in 45+ countries and treated 450,000+ patients.
This article has been adapted from "Targeting cancers", which originally appeared in the print edition of Ingenia 65 (December 2015).
Contributors
Dr Eric Mayes FRSC, Endomag CEO, has nearly two decades of experience in leading materials technology companies. He was named the Royal Society of Chemistry’s ‘Entrepreneur of the Year 2003’. A US-UK dual national, he holds a BSc in Physics from Arkansas State University and a PhD in Chemistry from the University of Bath.
Keep up-to-date with Ingenia for free
SubscribeRelated content
Health & medical

Kidney dialysis
Small haemodialysis machines have been developed that will allow more people to treat themselves at home. The SC+ system that has been developed is lighter, smaller and easier to use than existing machines.

Engineering polymath wins major award
The 2015 Queen Elizabeth Prize for Engineering has been awarded to the ground-breaking chemical engineer Dr Robert Langer FREng for his revolutionary advances and leadership in engineering at the interface between chemistry and medicine.
Blast mitigation and injury treatment
The Royal British Legion Centre for Blast Injury Studies is a world-renowned research facility based at Imperial College London. Its director, Professor Anthony Bull FREng, explains how a multidisciplinary team is helping protect, treat and rehabilitate people who are exposed to explosive forces.
Transcranial magnetic stimulation
Magnetic stimulation, especially of specific regions of the brain, has added greatly to the understanding of normal brain function and is being increasingly used to stimulate nerves for therapy. Mike Polson, Engineering Director of Magstim Company Ltd, talks about the history and potential of transcranial magnetic stimulation.
Other content from Ingenia
Quick read

- Environment & sustainability
- Opinion
A young engineer’s perspective on the good, the bad and the ugly of COP27

- Environment & sustainability
- Issue 95
How do we pay for net zero technologies?
Quick read

- Transport
- Mechanical
- How I got here
Electrifying trains and STEMAZING outreach

- Civil & structural
- Environment & sustainability
- Issue 95